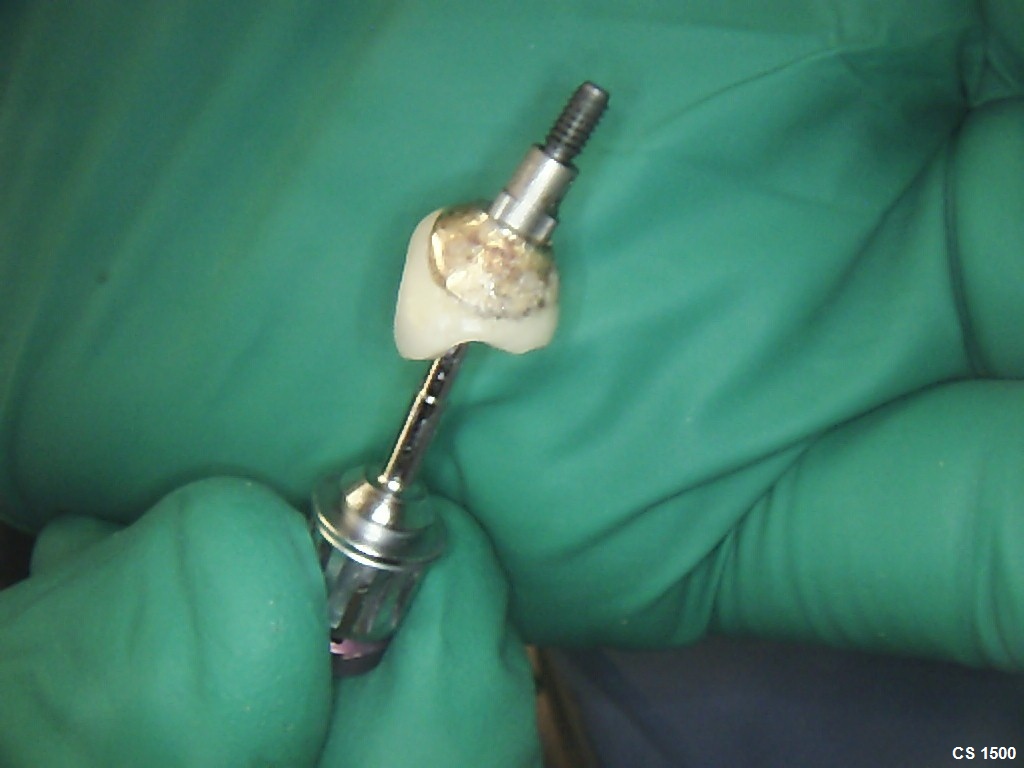
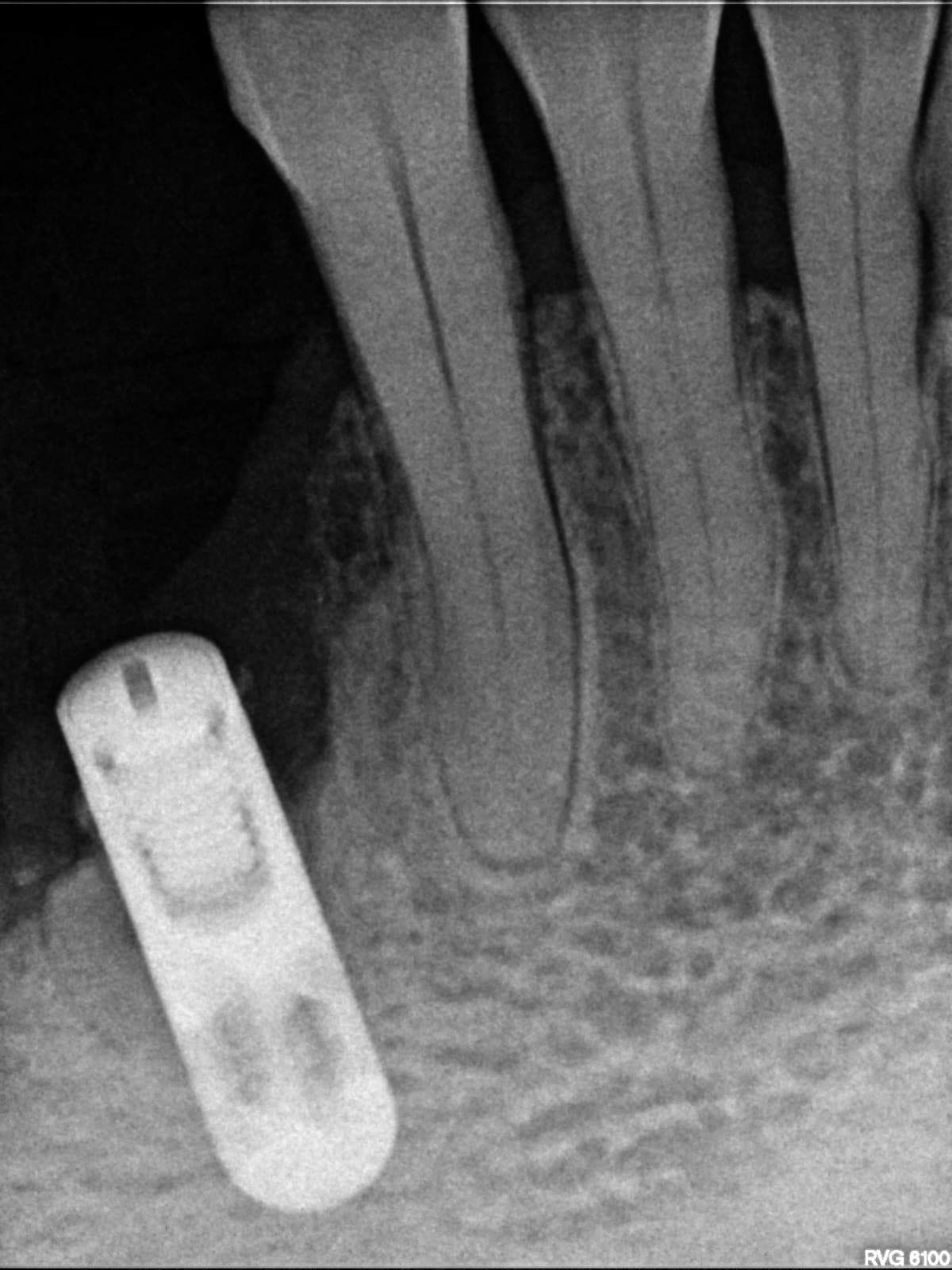
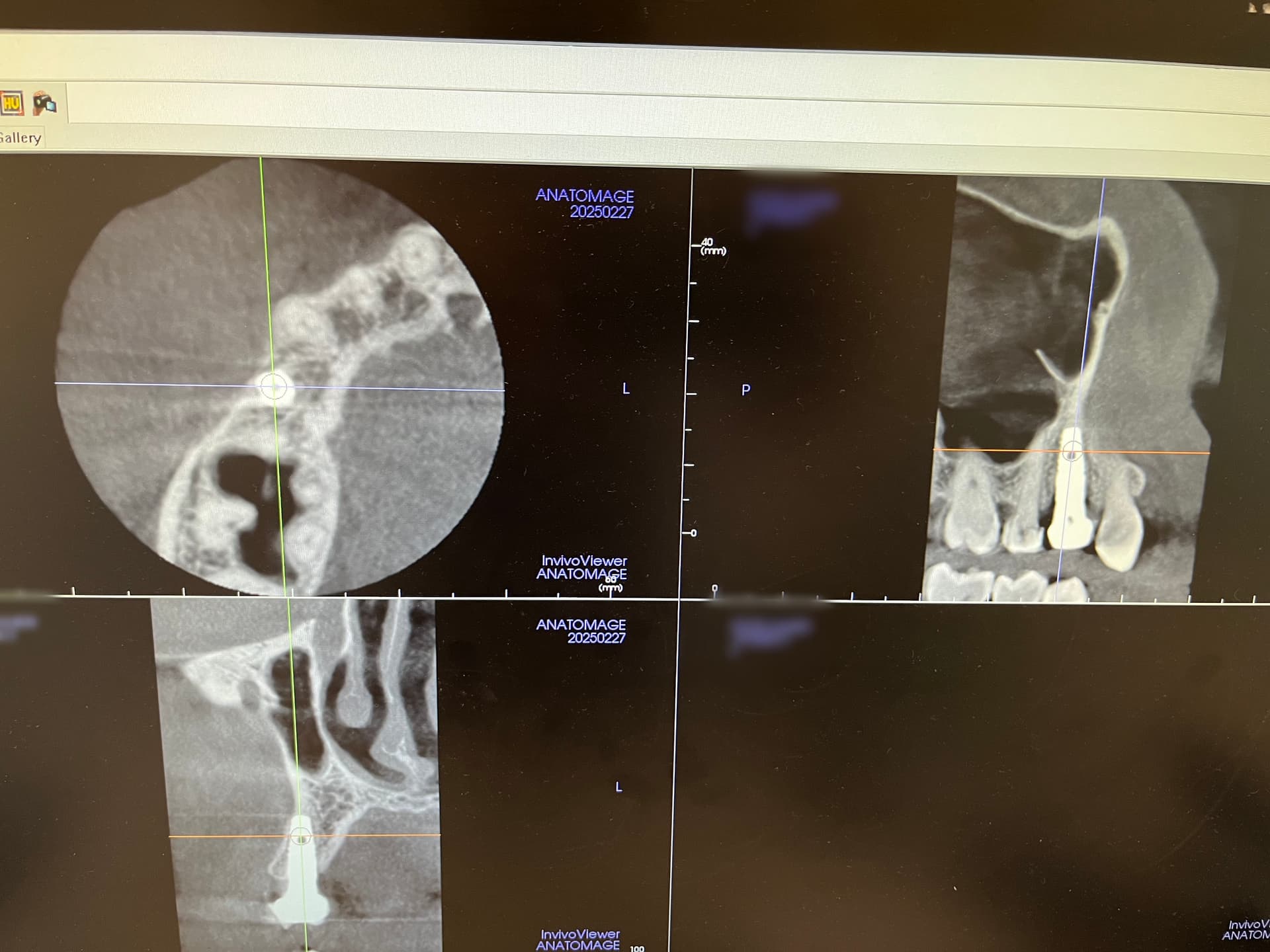
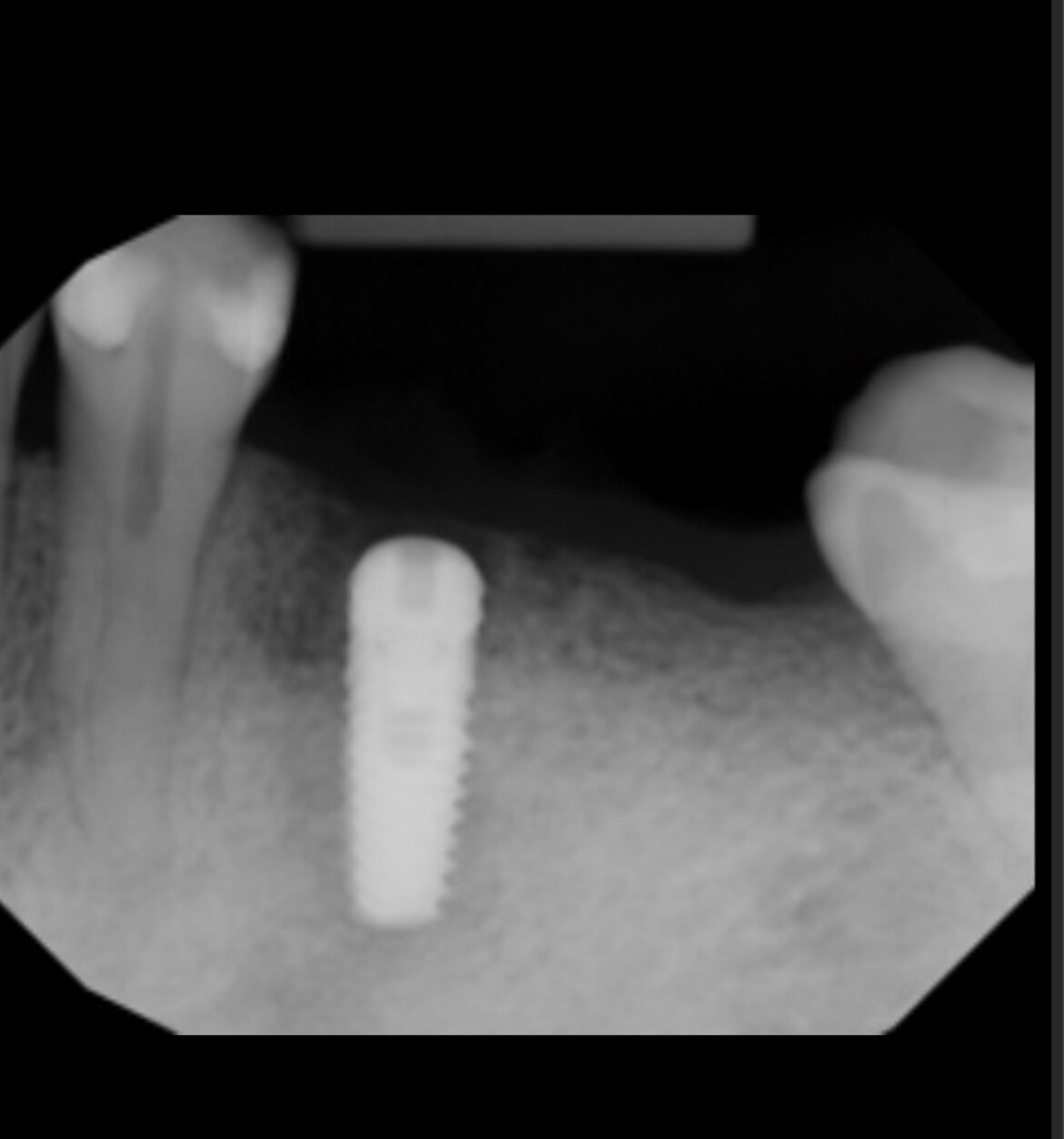
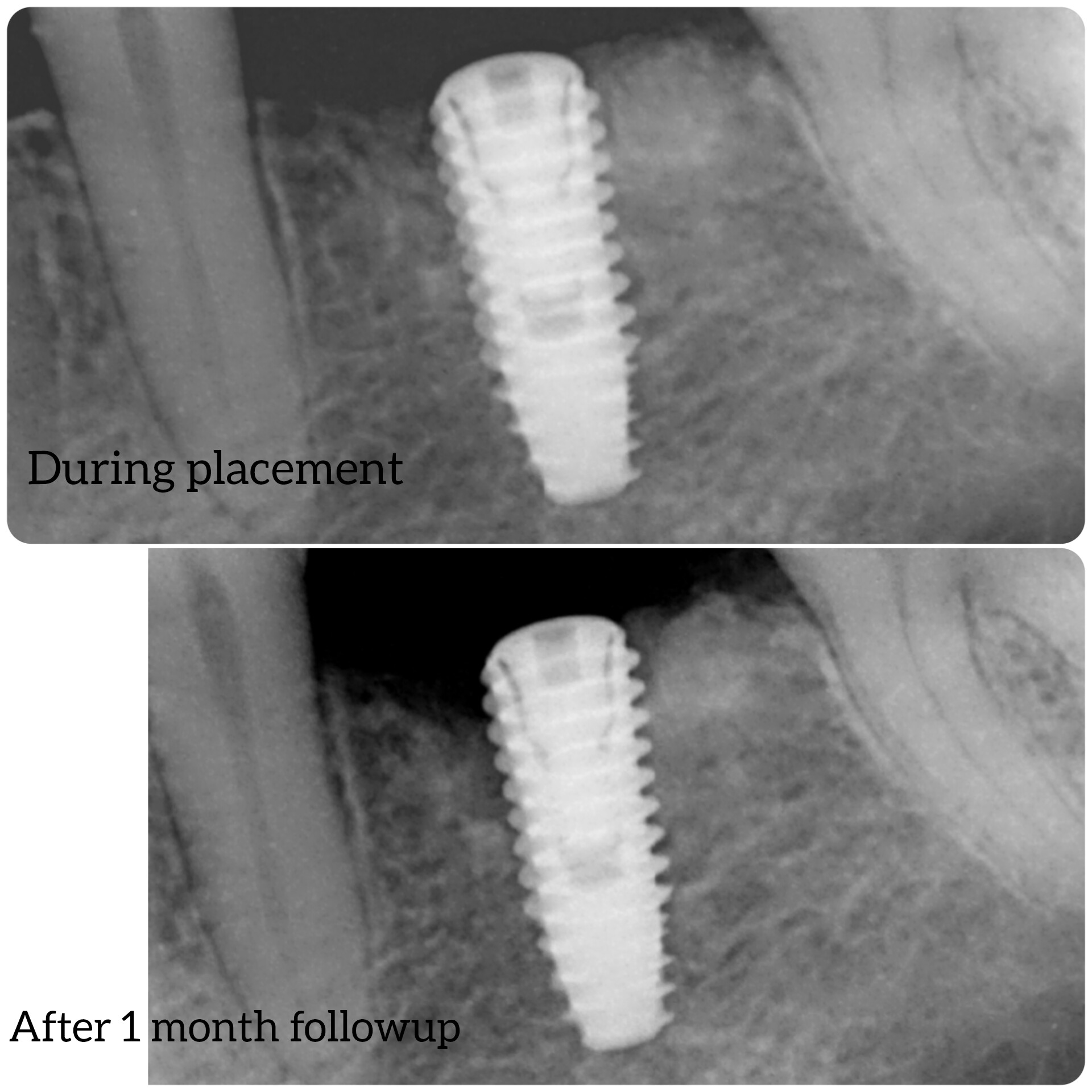
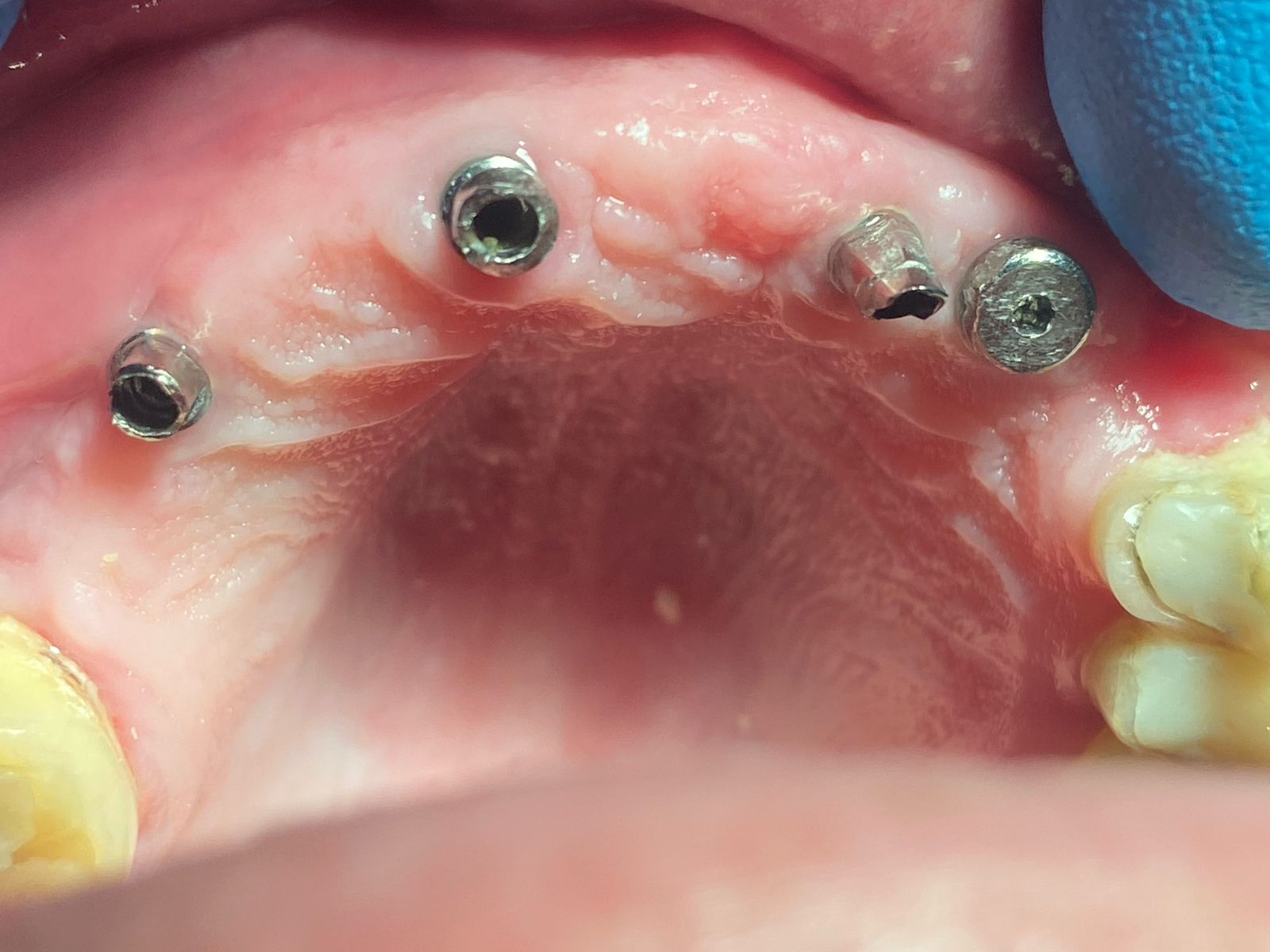
Would you accept a non-labled abutment?
I ordered fixtures and abutments from my dealer. He delivered the fixtures and then later delivered the abutments. The problem is that the abutments were delivered unpackaged and without any label. Can I accept it? What would you do?

9 Comments on Would you accept a non-labled abutment?
New comments are currently closed for this post.
Allen
1/4/2016
Medical-Legally you should have a lot and reference number for these parts. I would not accept it, and tell him to take it back and bring you one with proper packaging
Richard Hughes, DDS, FAAI
1/4/2016
I agree with Allen. It is necessary for completeness of record. The patient may move or transfer. Many reasons come arise that will require part name, part manufacturer, part ordering number and lot number.
David Bernhard
1/5/2016
Some implant warranty programs are voided with off label, third party components.
Alejandro Berg
1/5/2016
You are kidding right????? there is and can be only one answer to that and you know exactly what it is.
Alex
Dr. Gerald Rudick
1/5/2016
I have recently been to Israel and visited the Adin Dental Implant Company's factory. I was most impressed how even the most minute part is laser labelled with a lot number and other specific information. To read these number you would need to use high power magnification.... so that being said, a reputable implant manufacturer would have this type of equipment.... and if parts are not labelled, you have no idea where they are being made, what the metal is, and you are asking for problems........
Tuss
1/6/2016
Hi - you need traceability on everything plus as the dentist you are legally obliged to make sure the products/ devices meet all legal guideling. You have no idea if this is a "knock-off" from some dealer on ebay!
Bob Schneider
1/6/2016
I absolutely would not accept a non-labeled abutment. Many knockoffs are labeled "compatible with" which does not equate to actually fitting and have undergone long term testing. The other posts have already indicated use of these abutments will void the manufacturers warranty. Medical-legal records are critical in the clinician being knowledgeable in what components they are placing in the patients mouth. This is mandated by the FDA implantable devices guidelines.
Andrey
1/6/2016
dear colleagues just one question
do prosthetic parts of implant qualify as implantable device in your country?
are you obliged to write down all prosthetic parts on medical records?
Raul Mena
1/6/2016
It is illegal to sell Implants or abutments without proper packaging and labeling.
As a Doctor you are responsible for the health and welfare of the patient.
Material, Sterility, Labeling are critical and very important in order to provide proper treatment to your patient and for proper record keeping. It is also very important to be able to have traceability.
All the above are a requirement of FDA and ISO 13485 and MDD CE.
Raul R. Mena DMD
President Quantum Implants