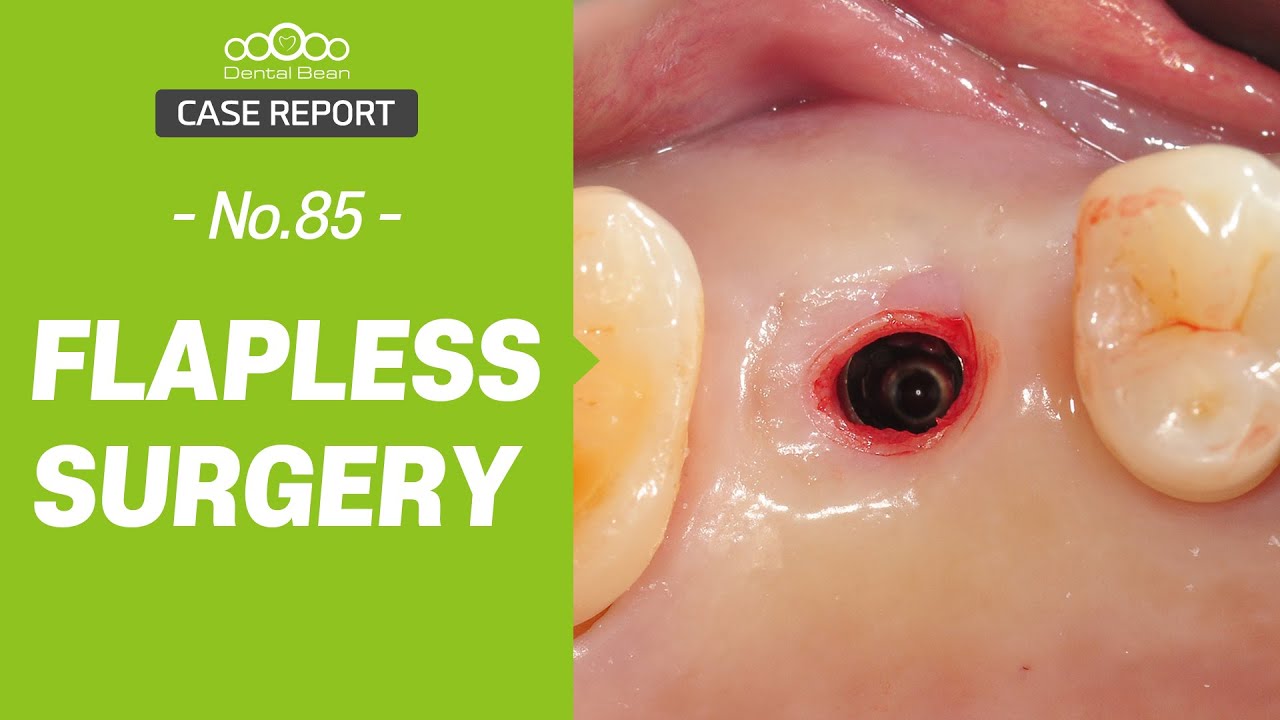
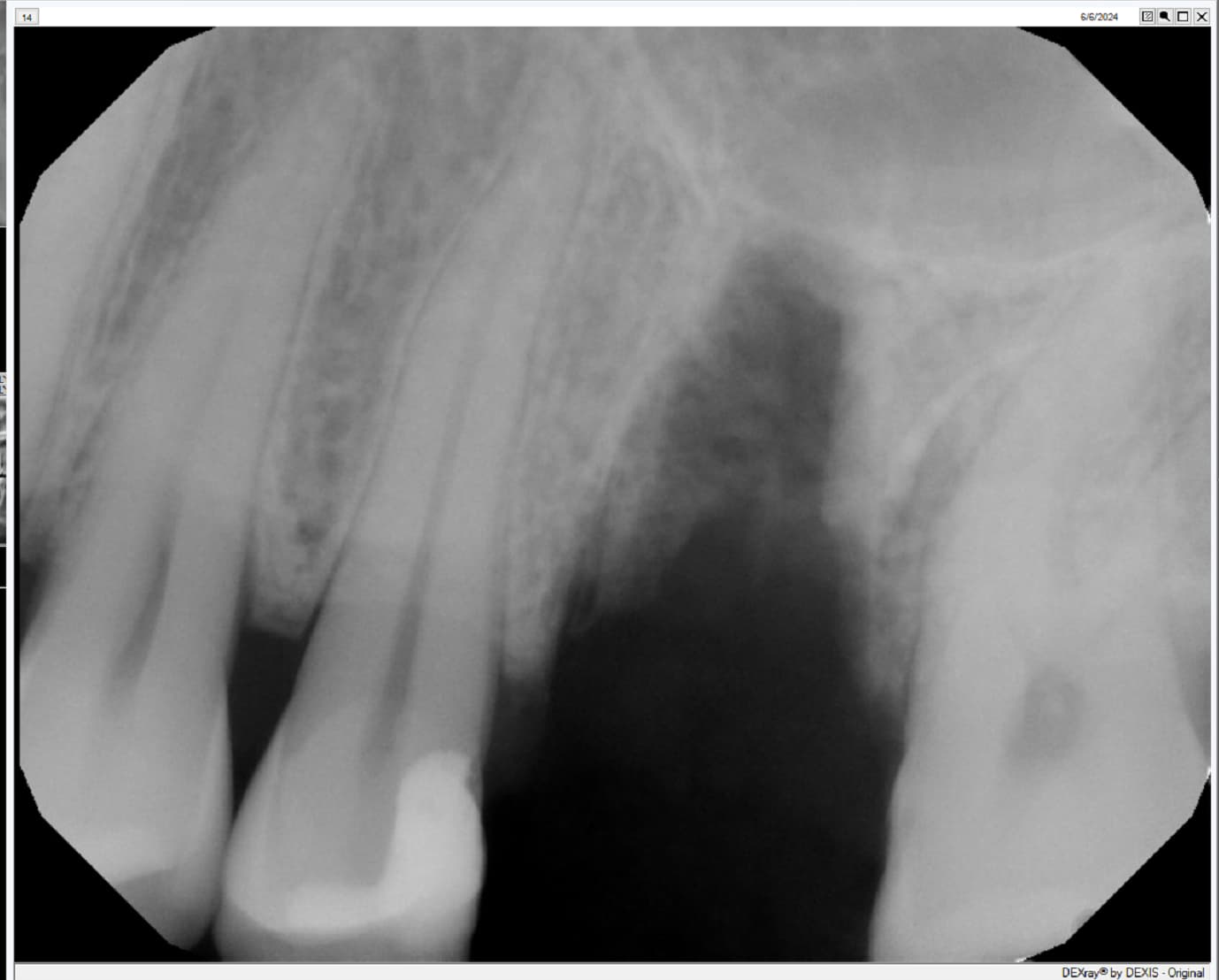
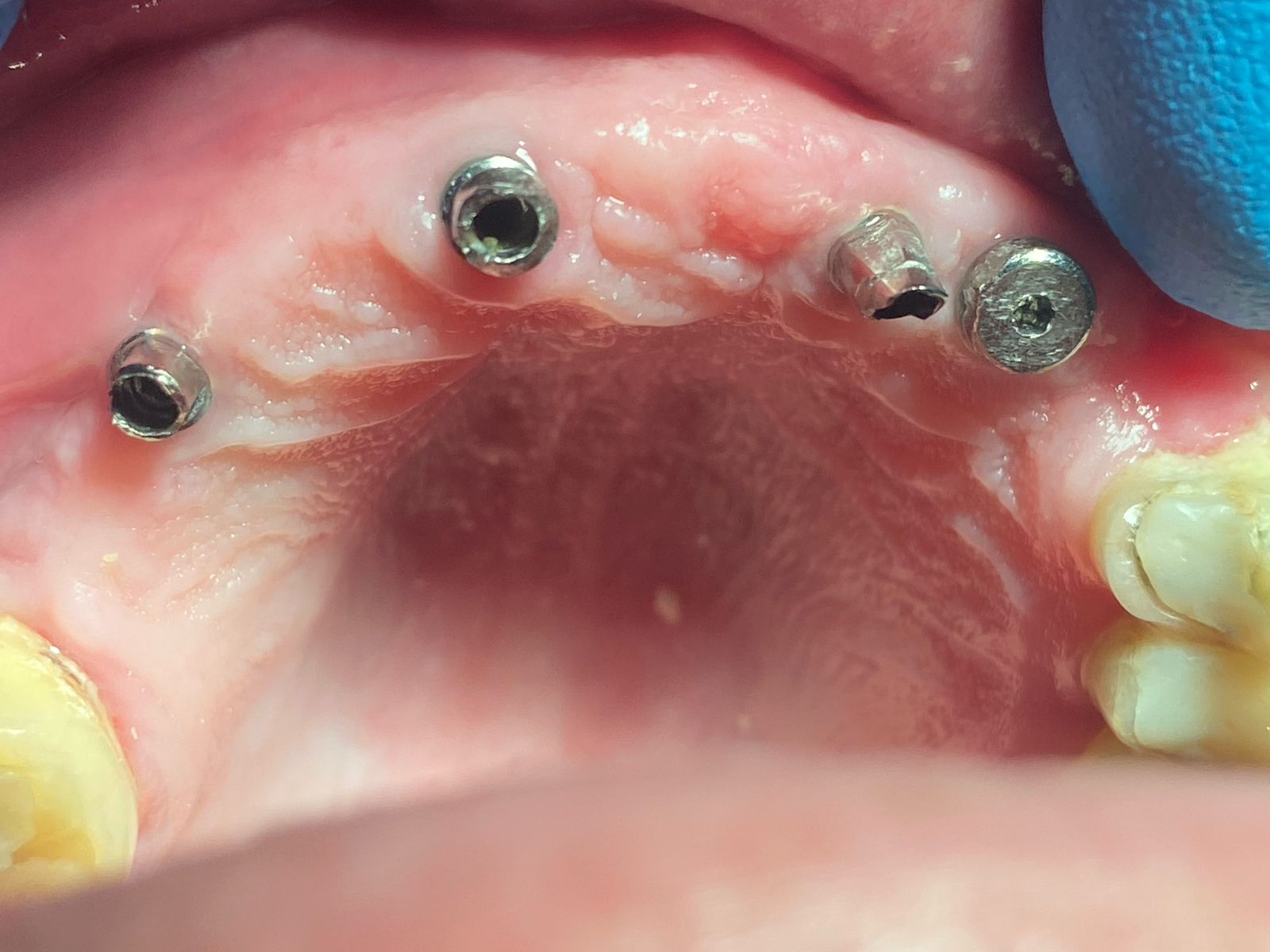
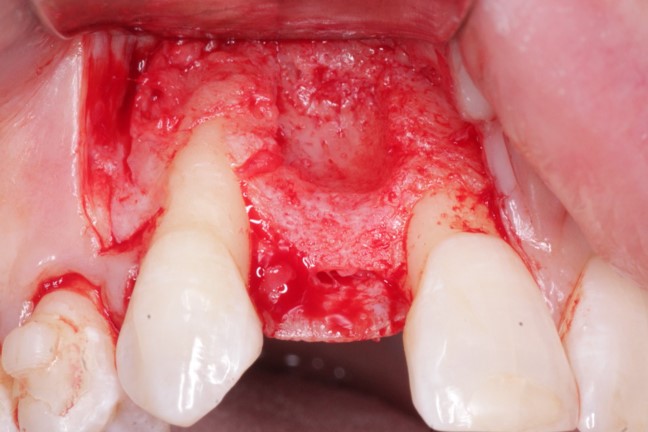
An Alternative to Bone Grafting?
Any thoughts on Medtronic’s Infuse product which an FDA panel recently recommended for approval to help regrow bone in adults to provide an anchor for dental implants?
Infuse is a collagen sponge impregnated with a human bone growth factor. It is already approved for use in certain lower back procedures and treating some forms of severe leg fractures.
Medtronic wants to market Infuse as an alternative to bone grafting, which the company says is equally effective, but requires surgeons to take bone from elsewhere in the body to anchor a dental device in the mouth. However, experts complained that Medtronic did not sufficiently test Infuse in tooth extraction sockets.
“The data submitted is not rigorous enough,” said Janine Janosky, an assistant professor of biostatistics at the University of Pittsburgh and a member of the panel. Dr. Patters an FDA panel member and a professor of periodontology at
the University of Tennessee, said that bone grafts like the ones Infuse is meant to replace are not generally performed in tooth extraction patients, limiting Infuse’s attractiveness as an alternative to grafting surgeries for those patients.
The FDA panel of outside
experts recommended Infuse’s label highlight that it has not
been tested in molar extraction sites, or in the lower jaw,
where the majority of tooth extractions occur.