FDA on Mini Implants
Dennis, a dentist, asks:
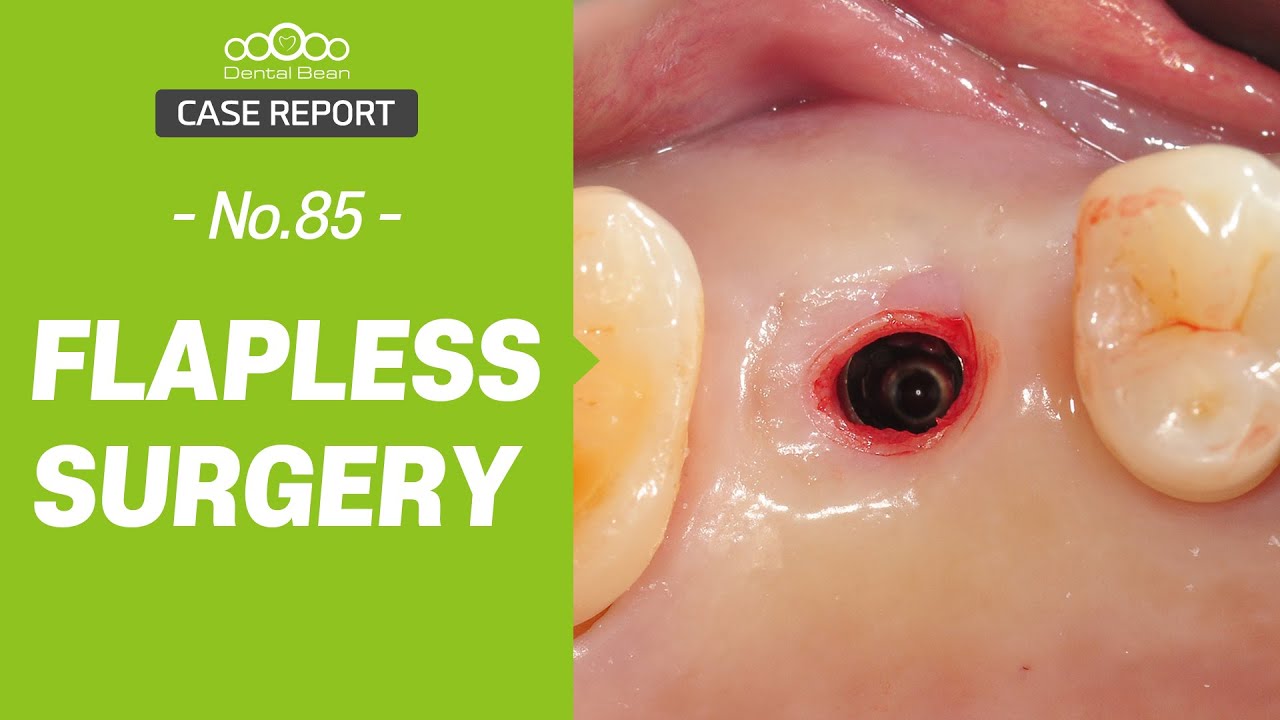
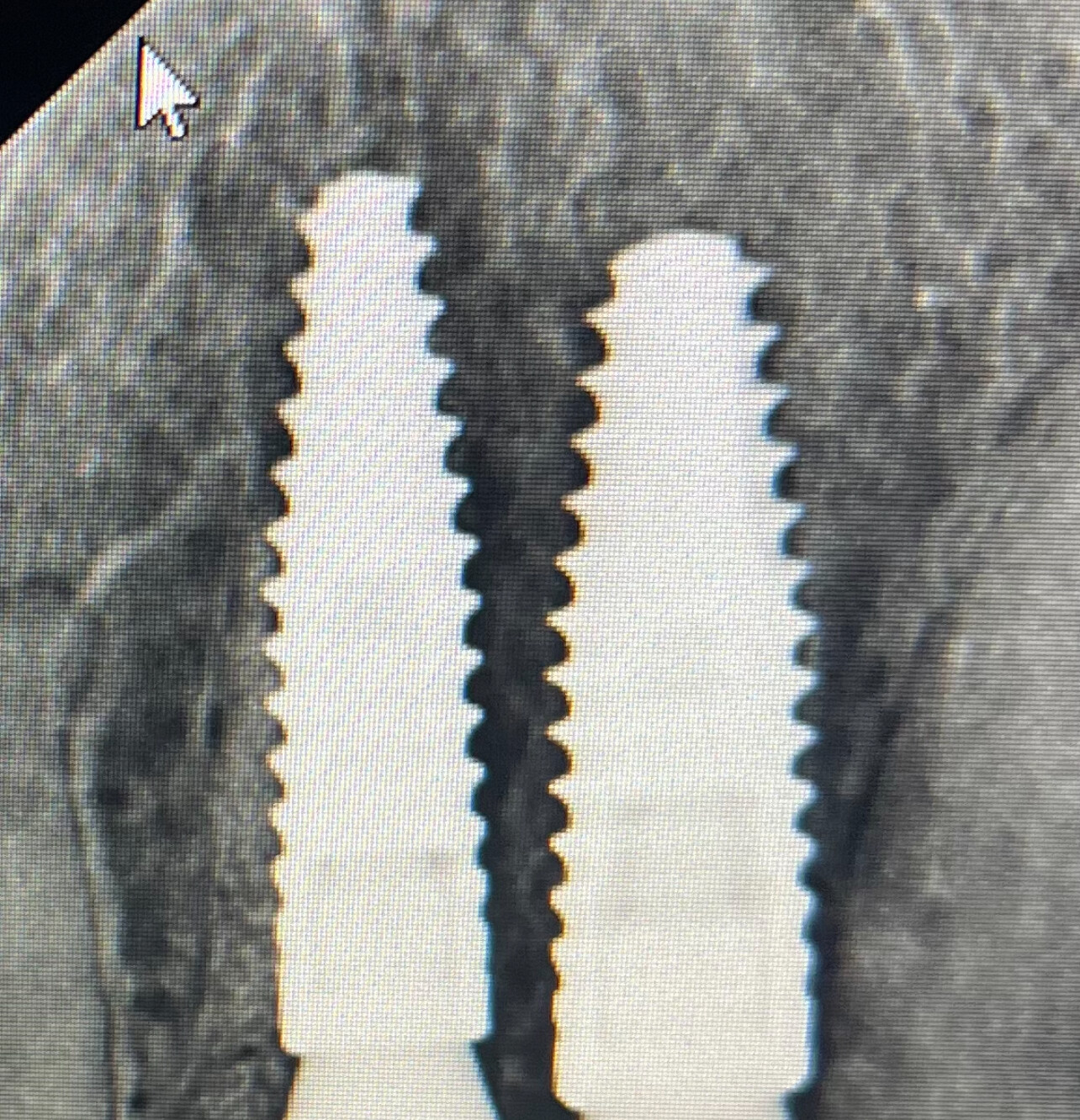
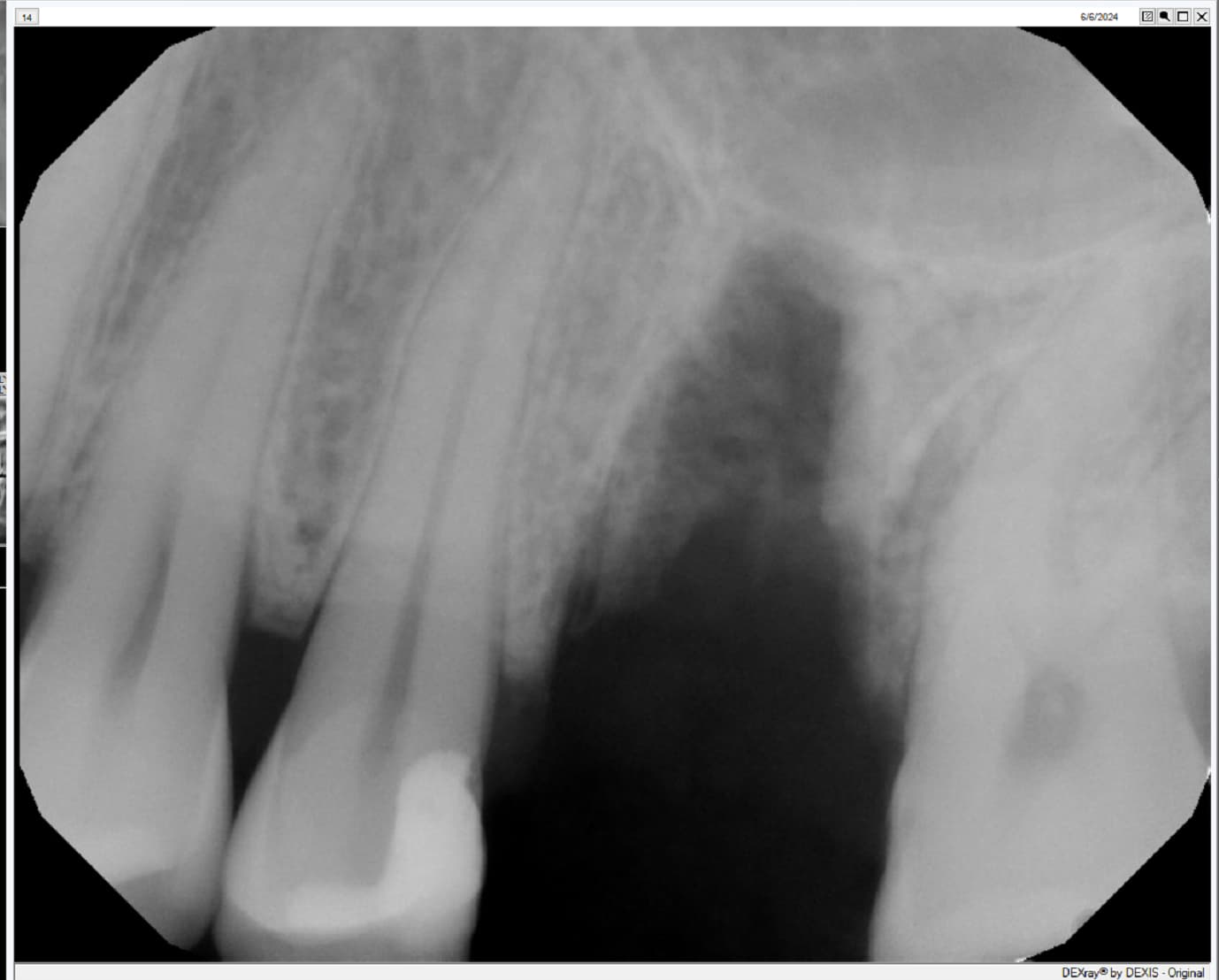
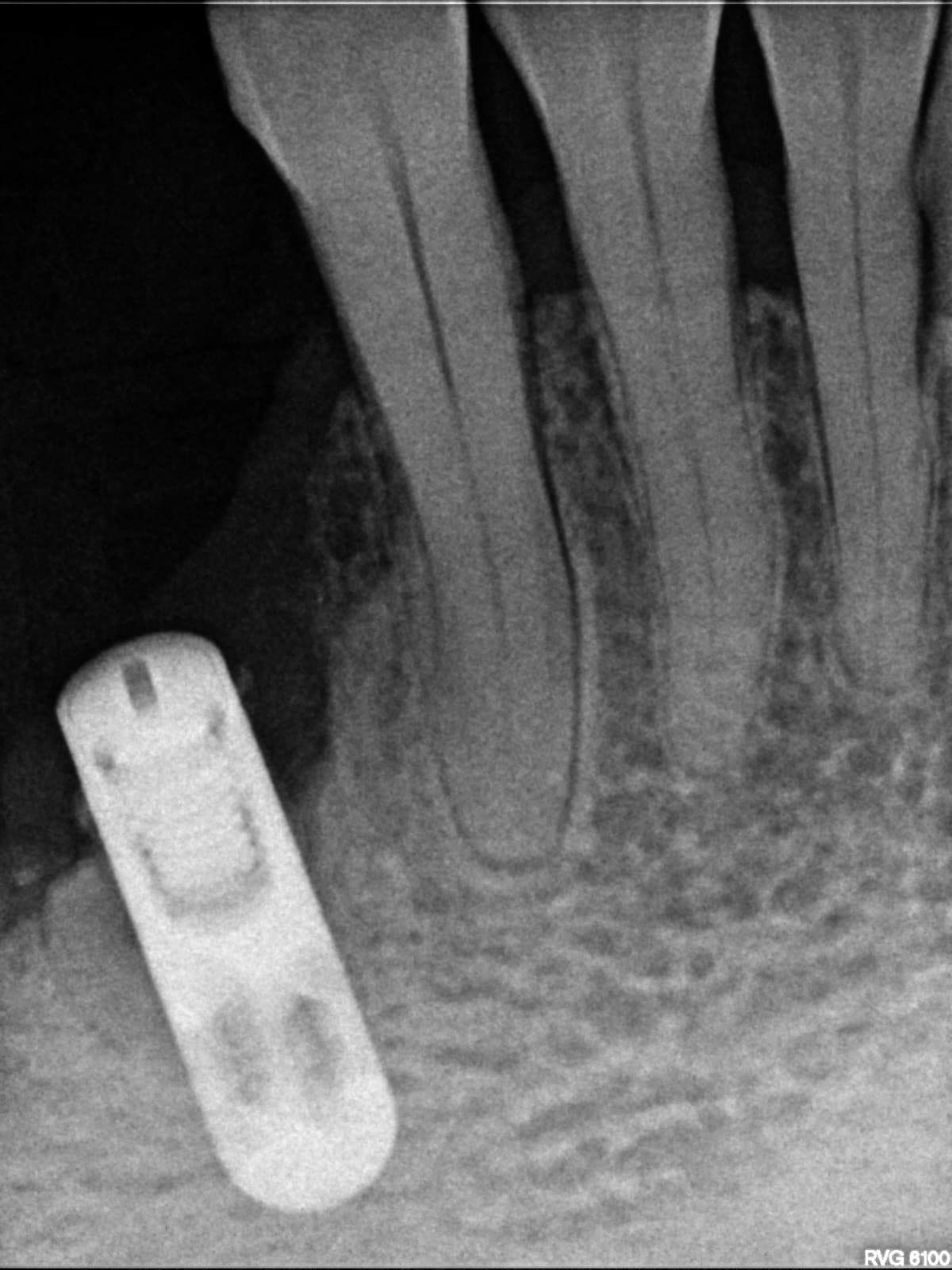
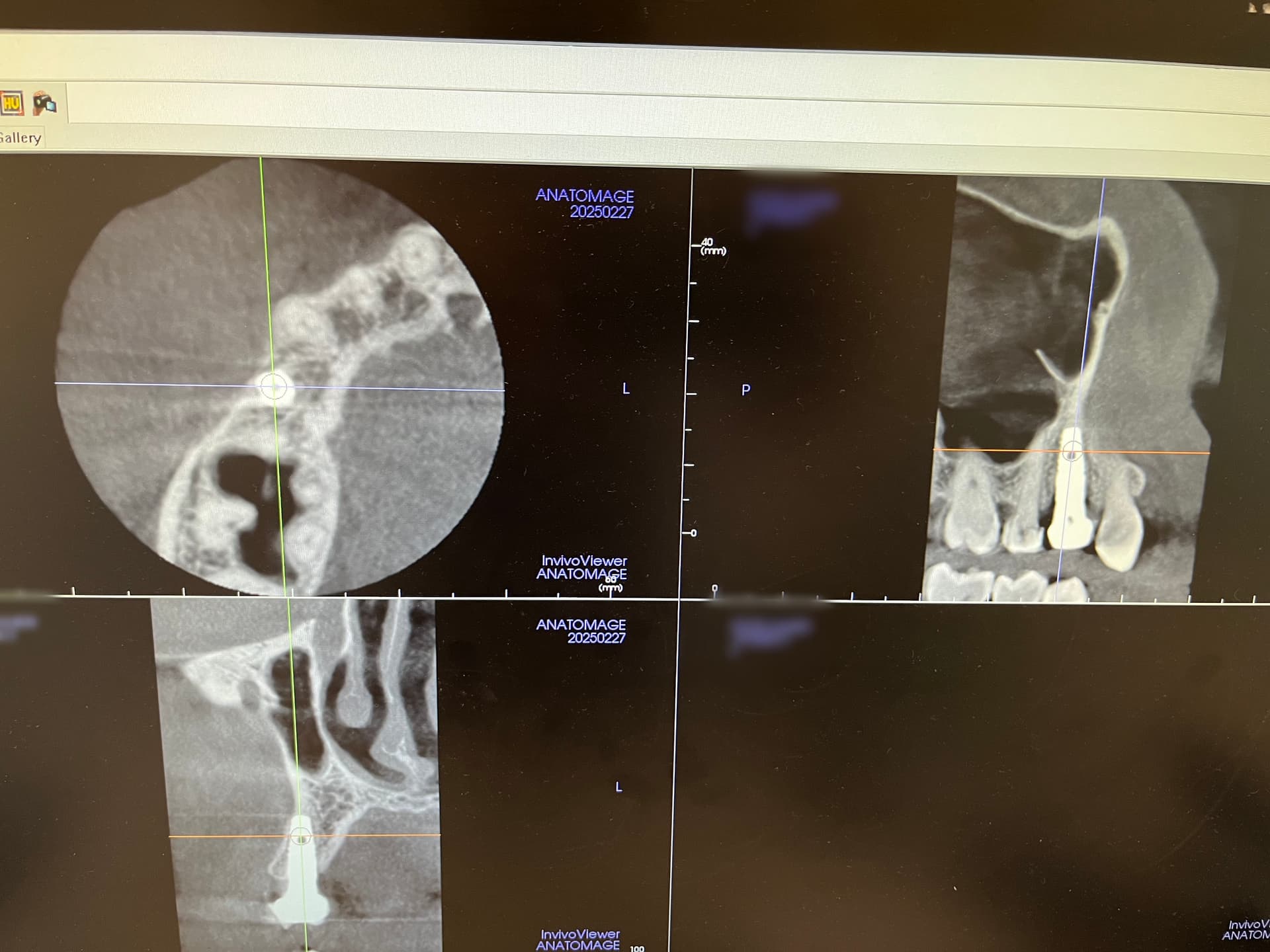
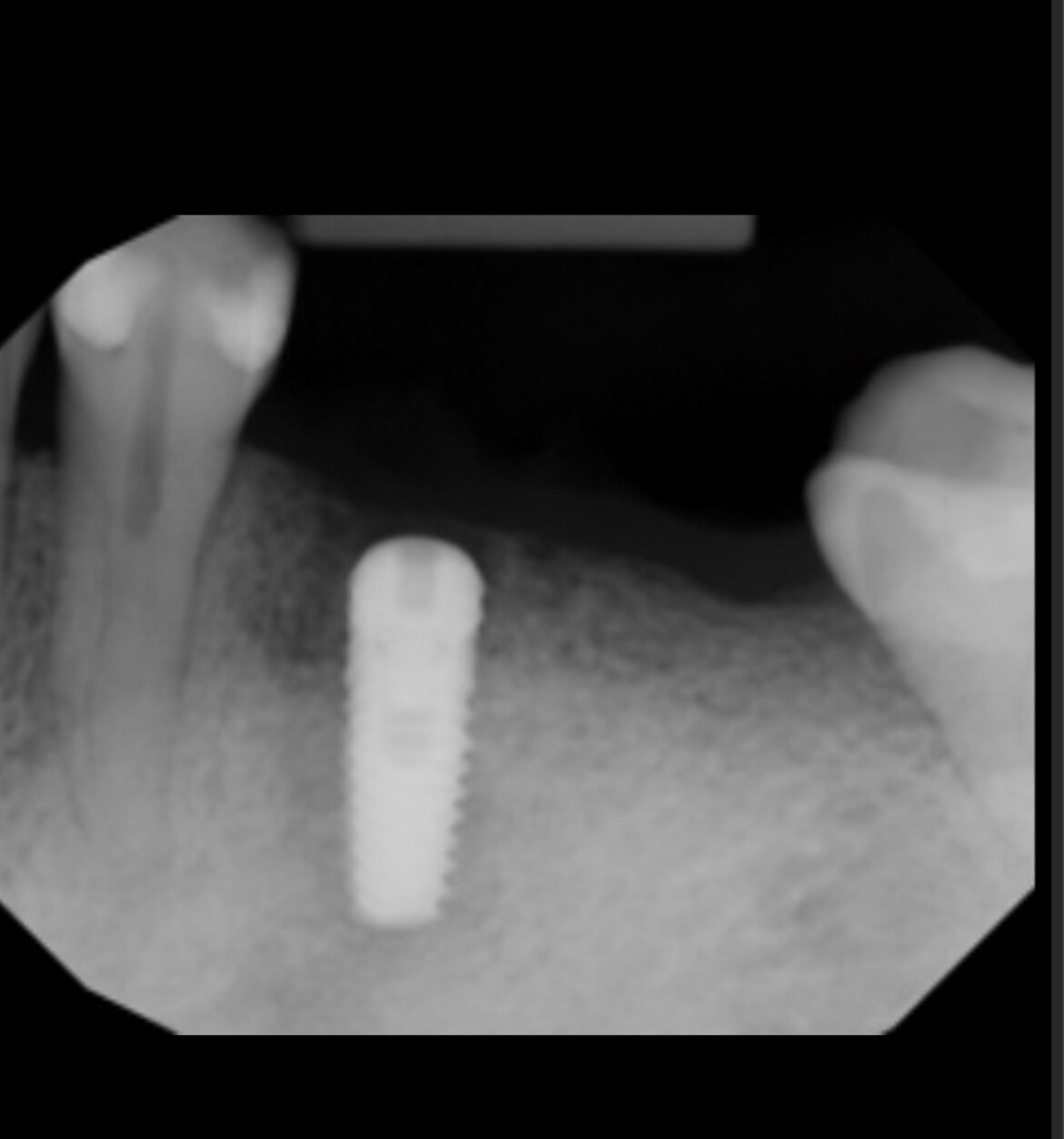
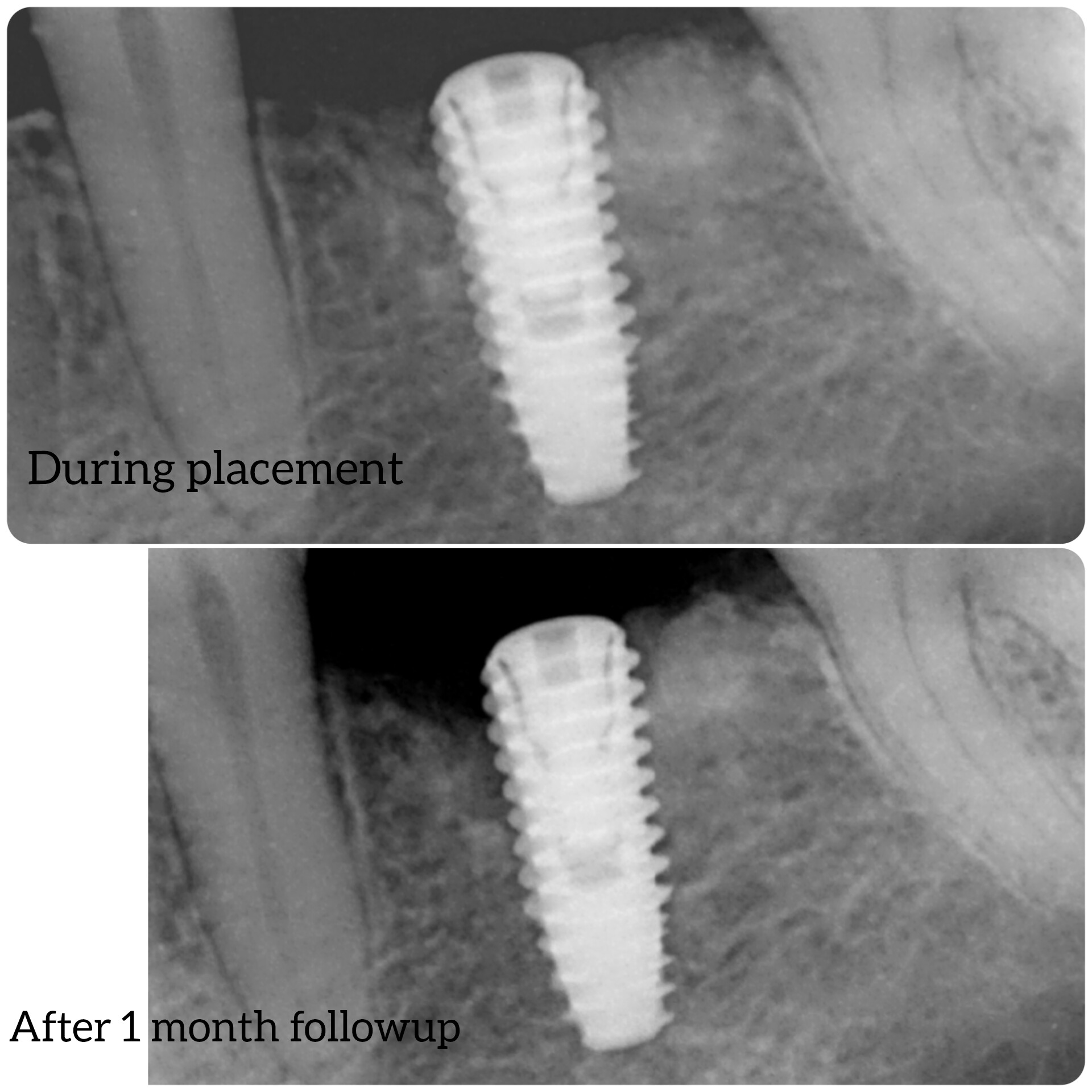
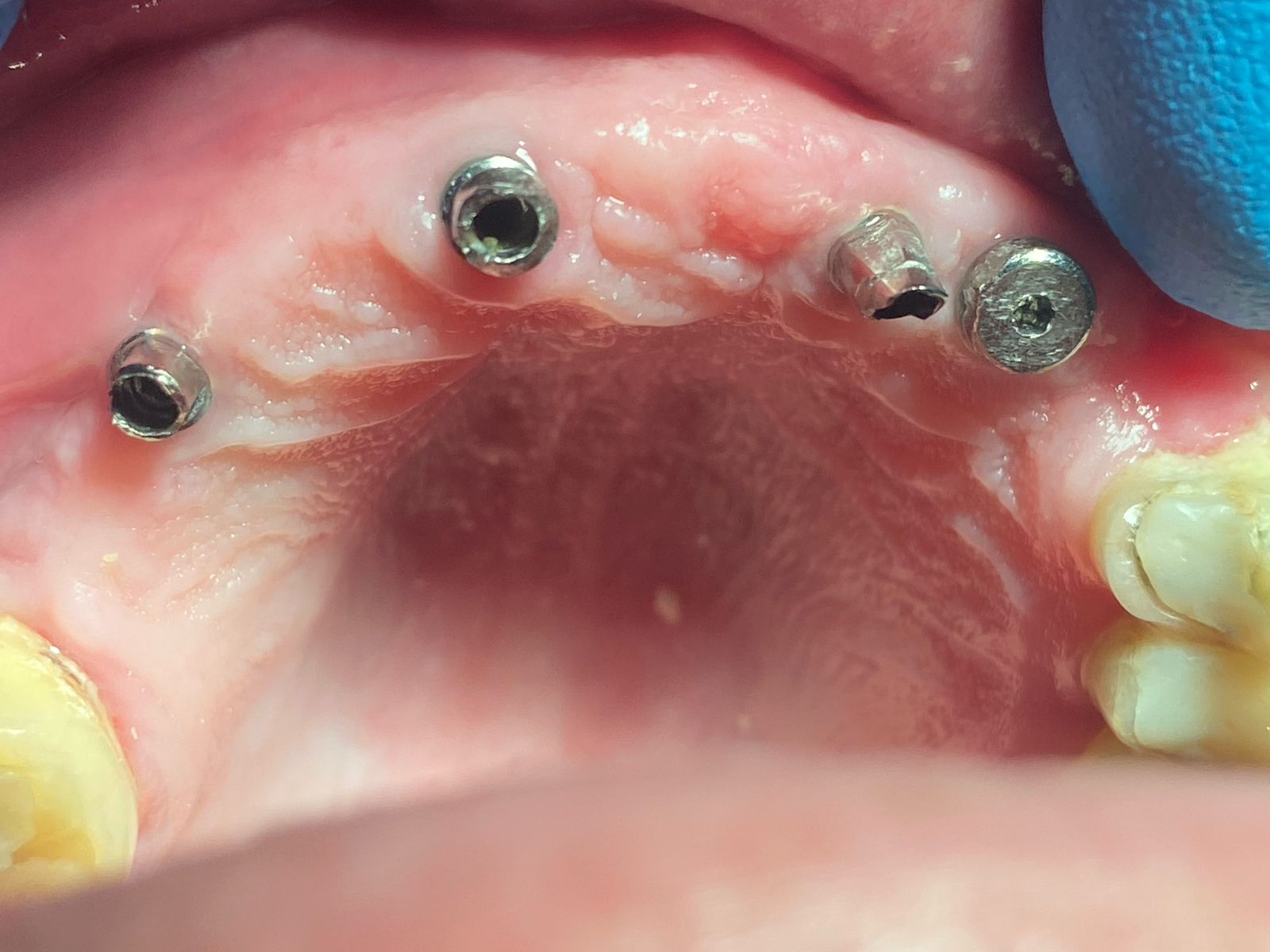
In all of the discussions on mini dental implants, there have been several references to the FDA requirement of a dental implant diameter of at least 3mm (formerly 3.25mm) and length of 7mm to qualify for the description of a dental implant.
1. When were these guidelines established? Does anybody have any reference material for this?
2. How does this ‘classification’ affect the insurance payments
(where allowable) of dental implants of either smaller diameter, such as mini
dental implants or shorter length, such as when Endopore and Bicon dental implants
are used?