Results Comparing Inion to Gore-Tex Announced
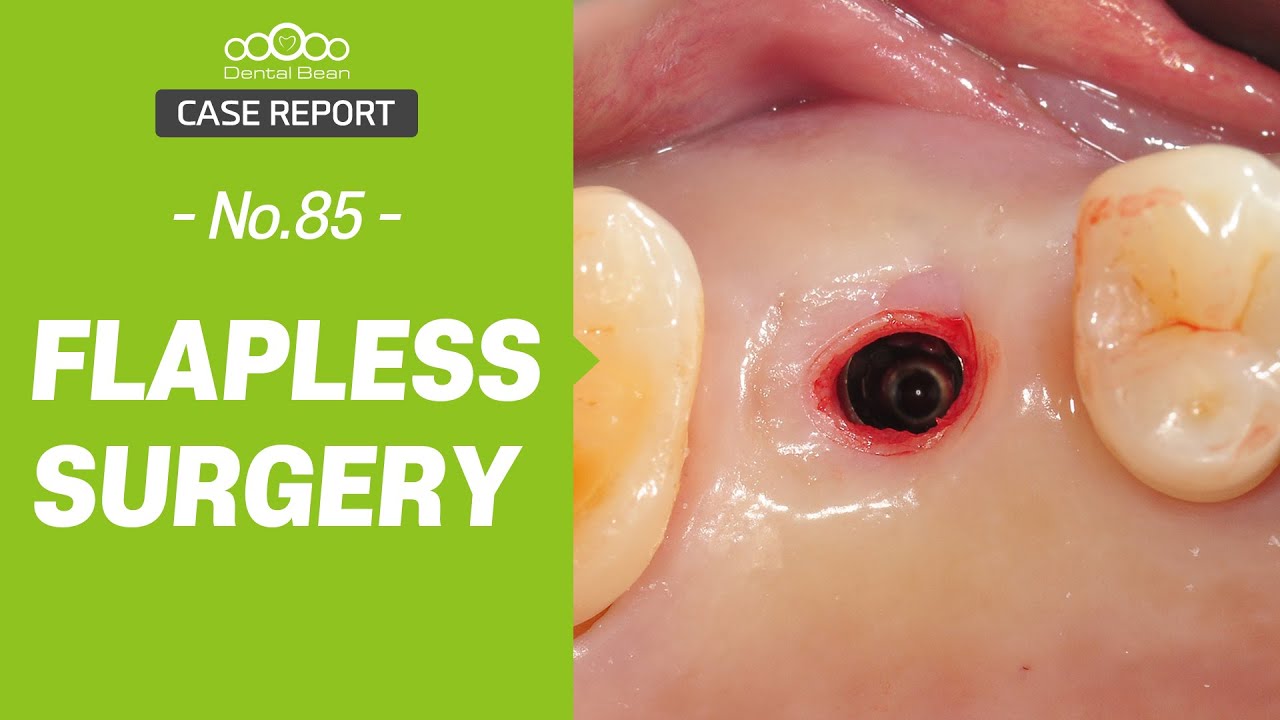
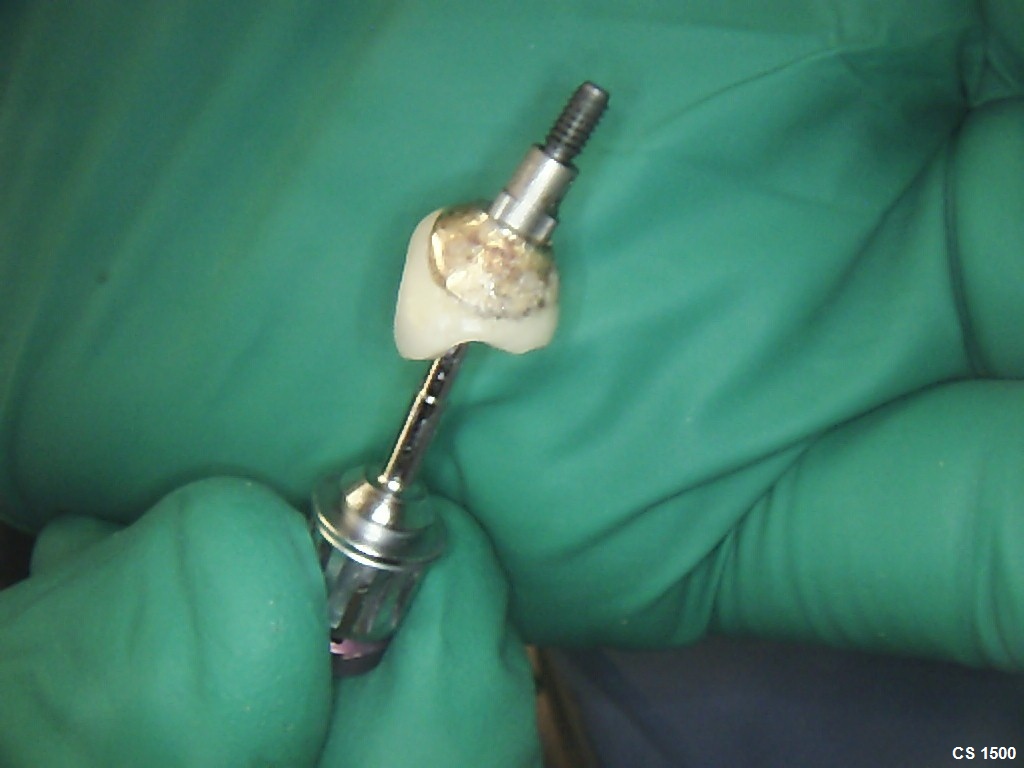
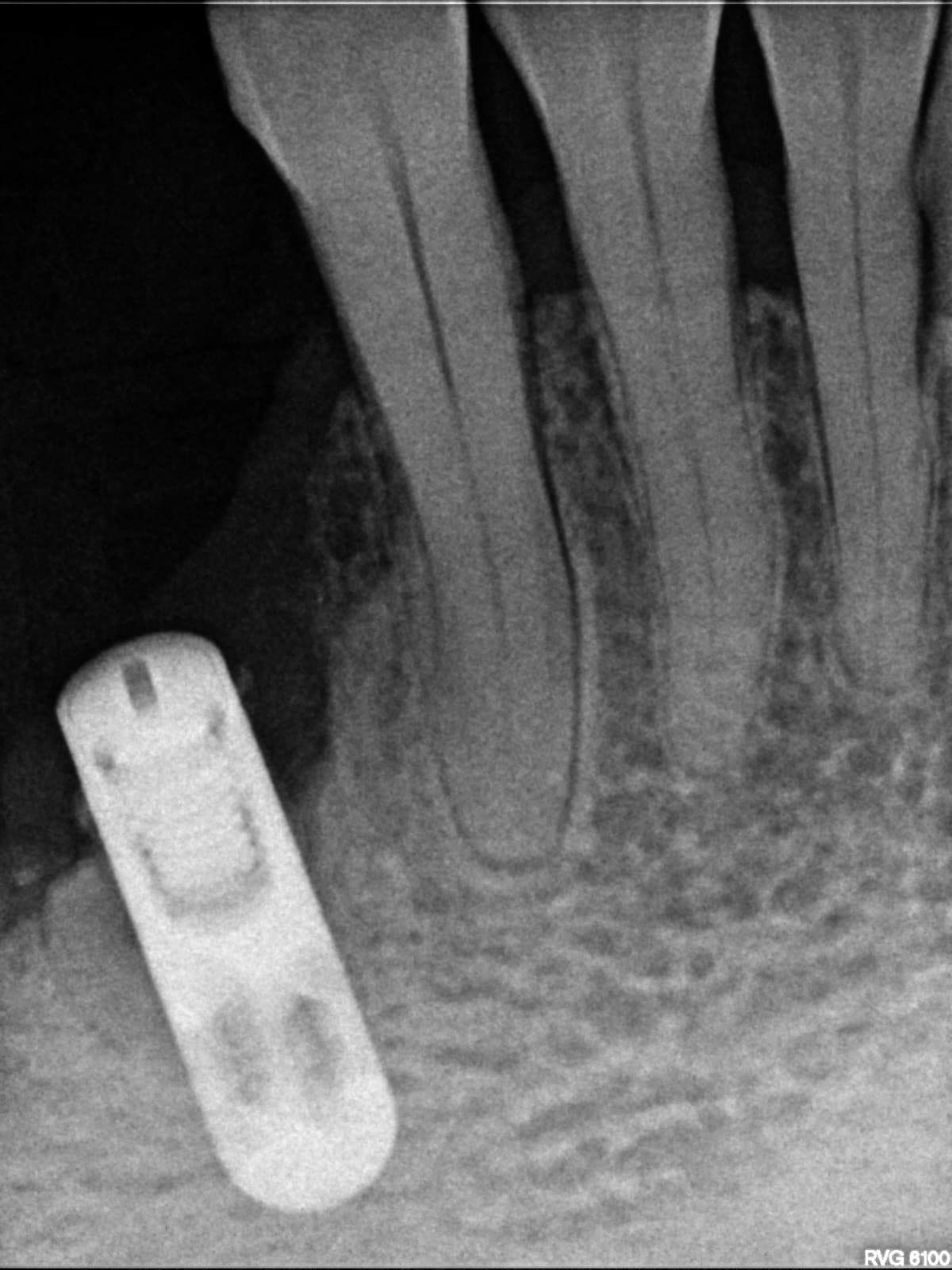
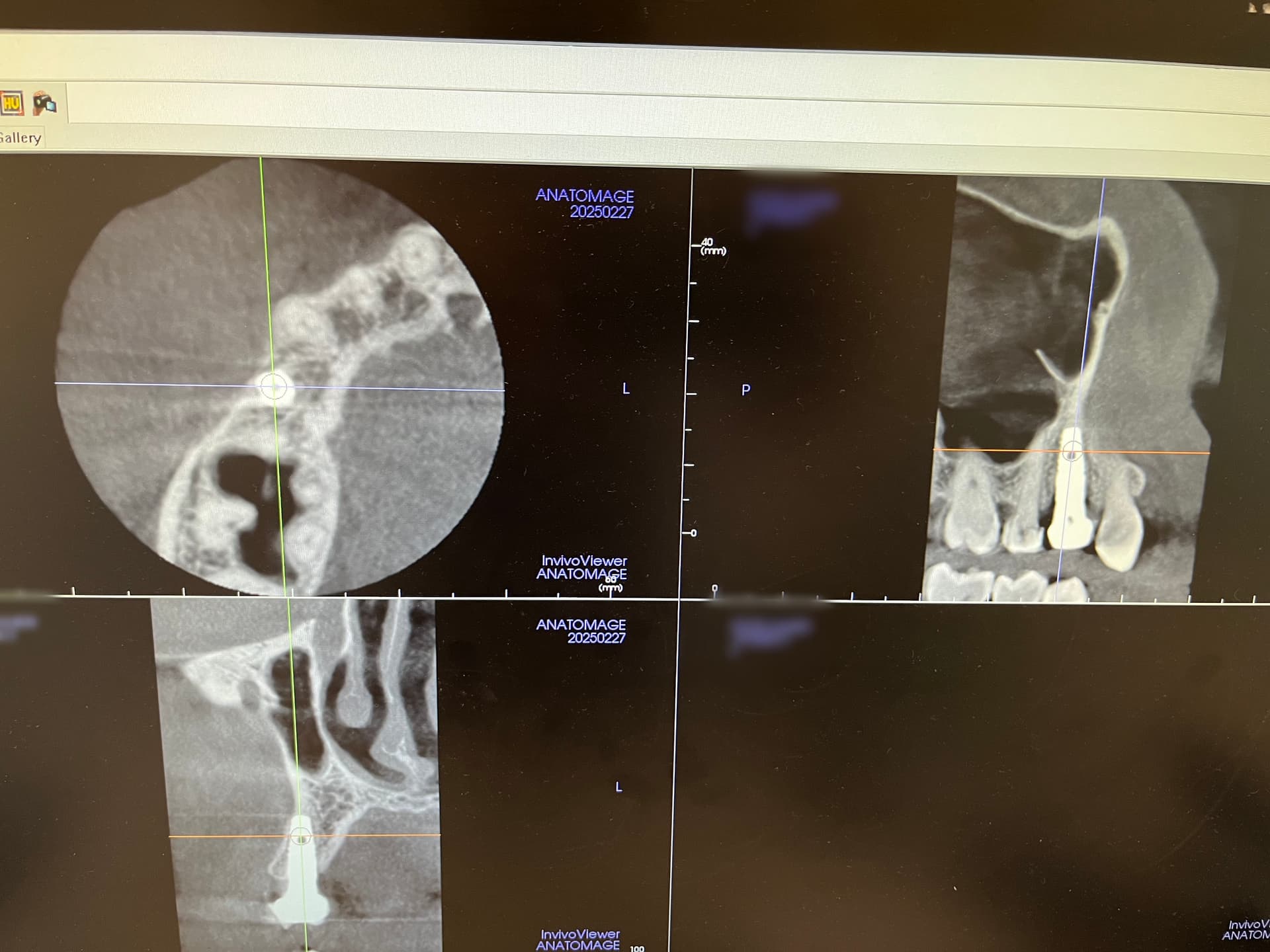
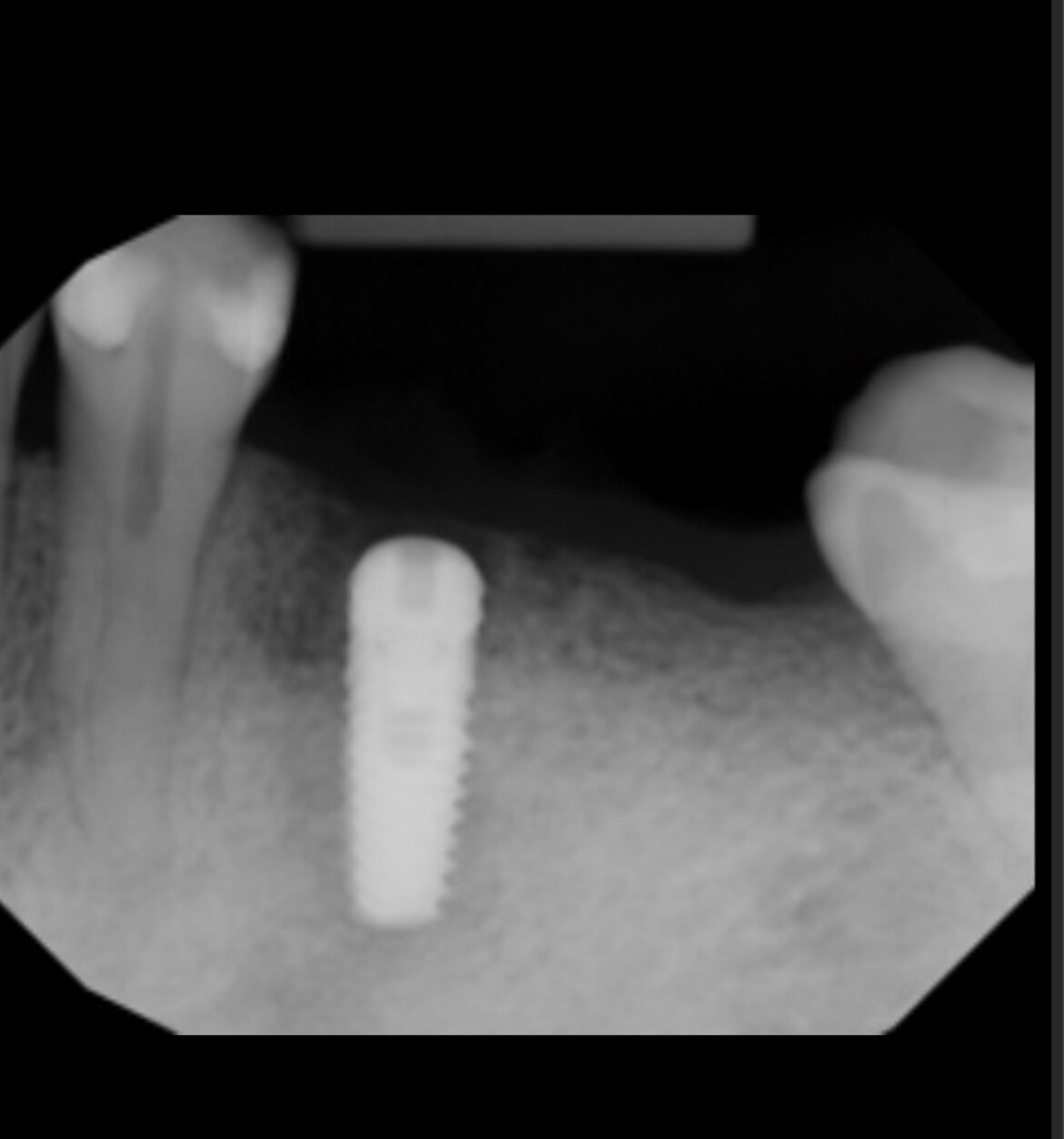
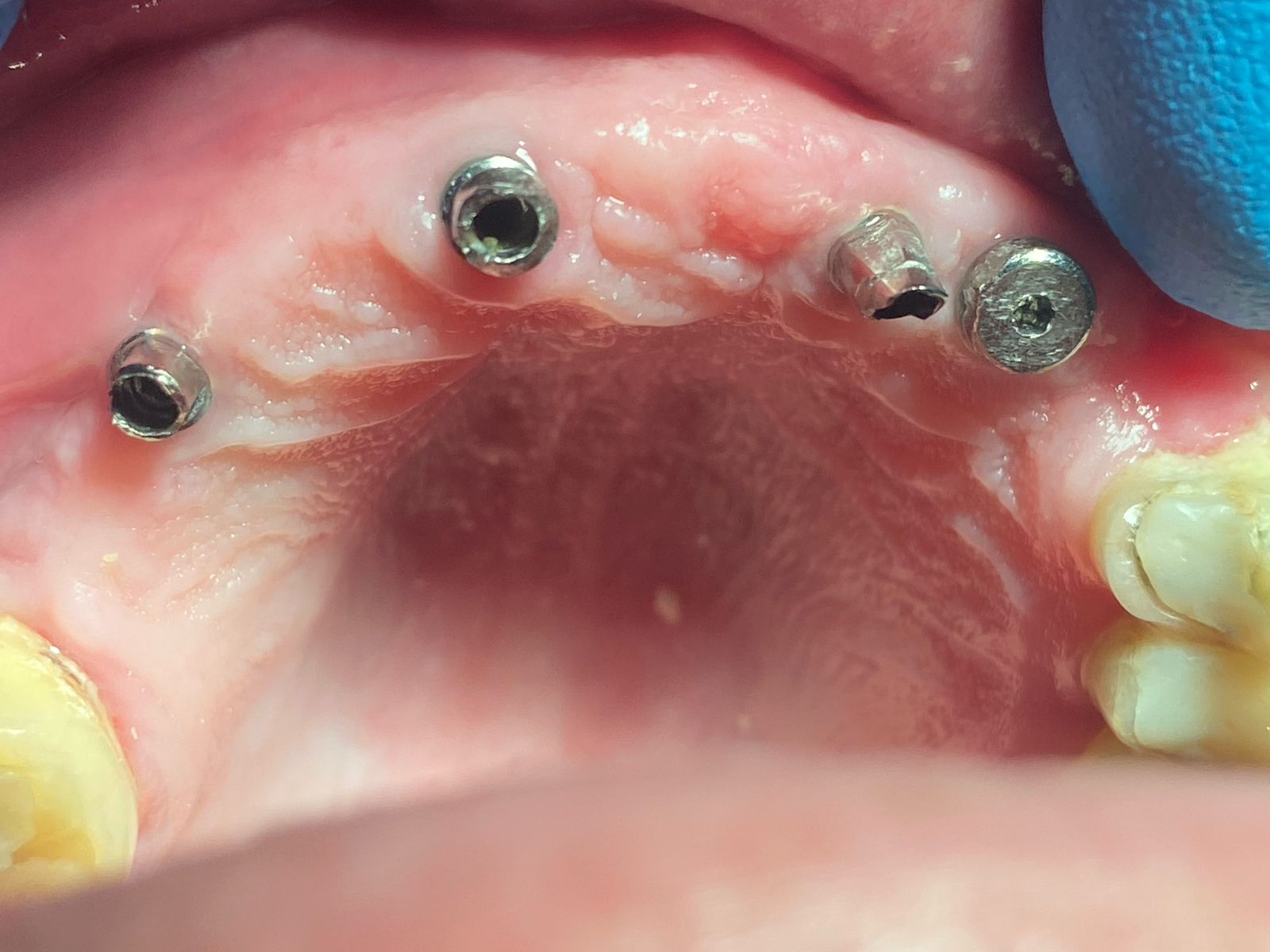
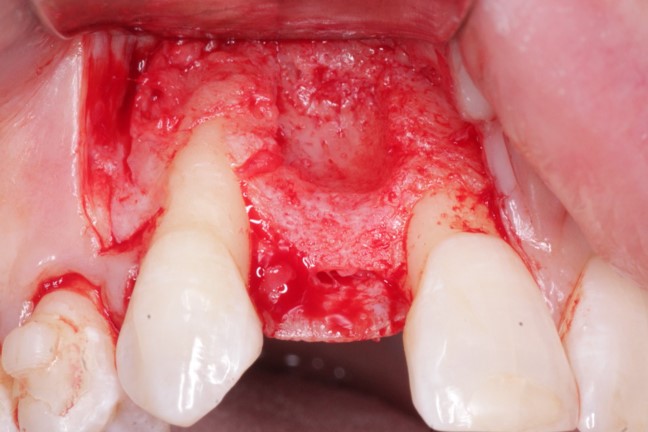
Inion (LSE: IIN.L), a UK company focused on the development and commercialisation of novel biodegradable medical implants, provided an update regarding the second clinical proof of principle study with Inion OptimaPLUS. The study was designed to evaluate the quality of new bone generated following a dental implant surgical procedure and compares Inion OptimaPLUS with a market-leading, non-biodegradable competitor product (GORE-TEX Regenerative Membrane Titanium Reinforced).
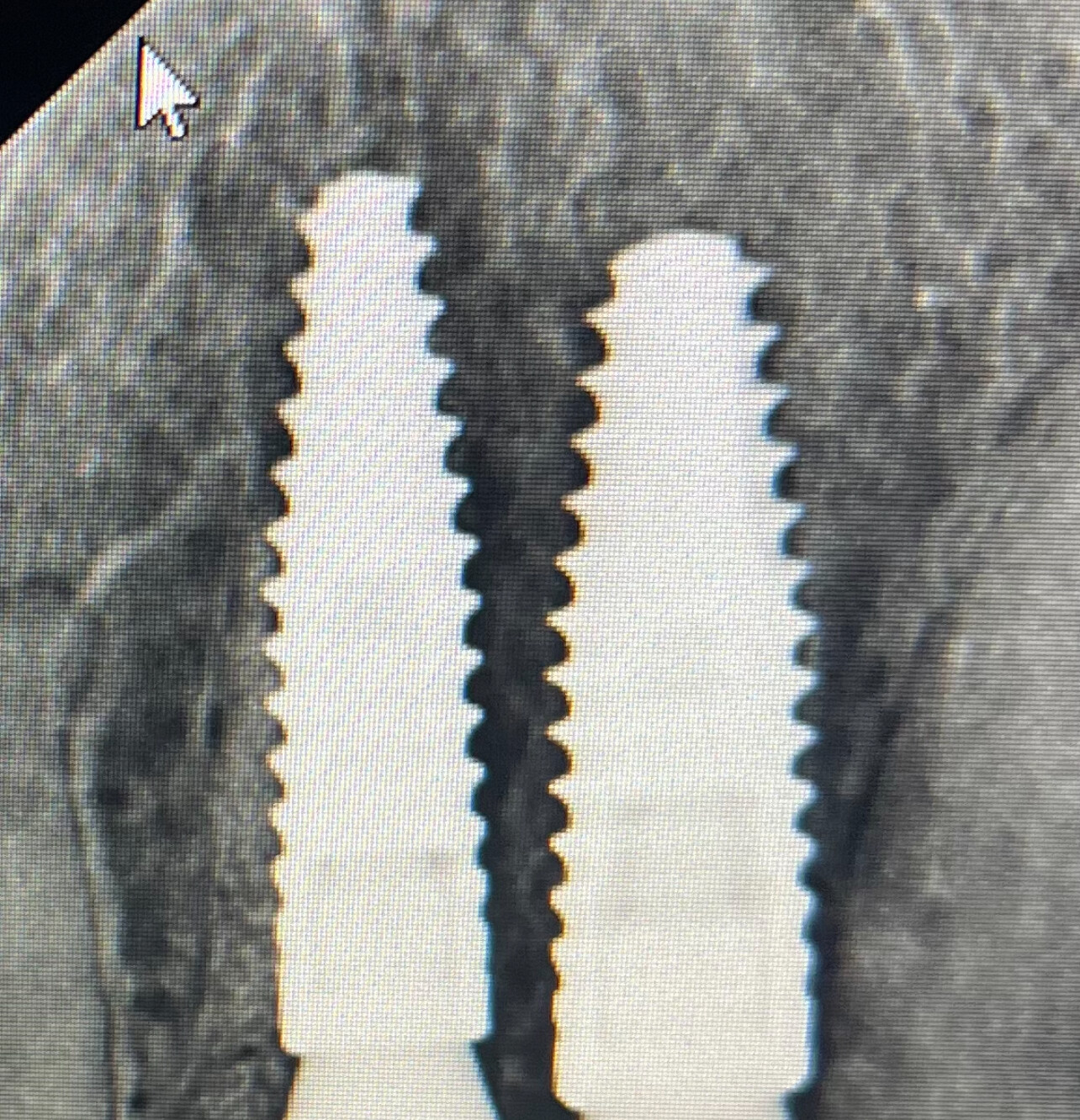
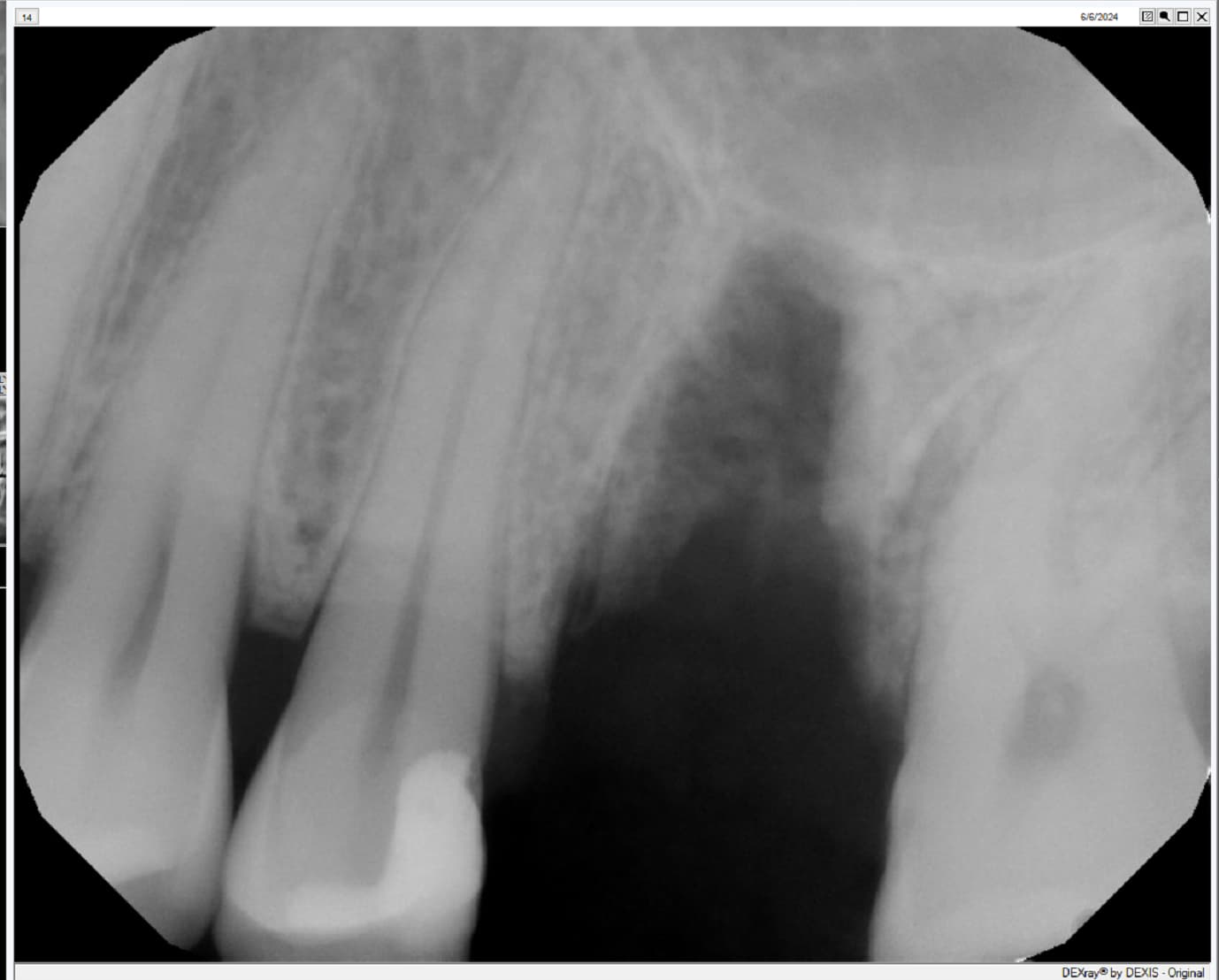
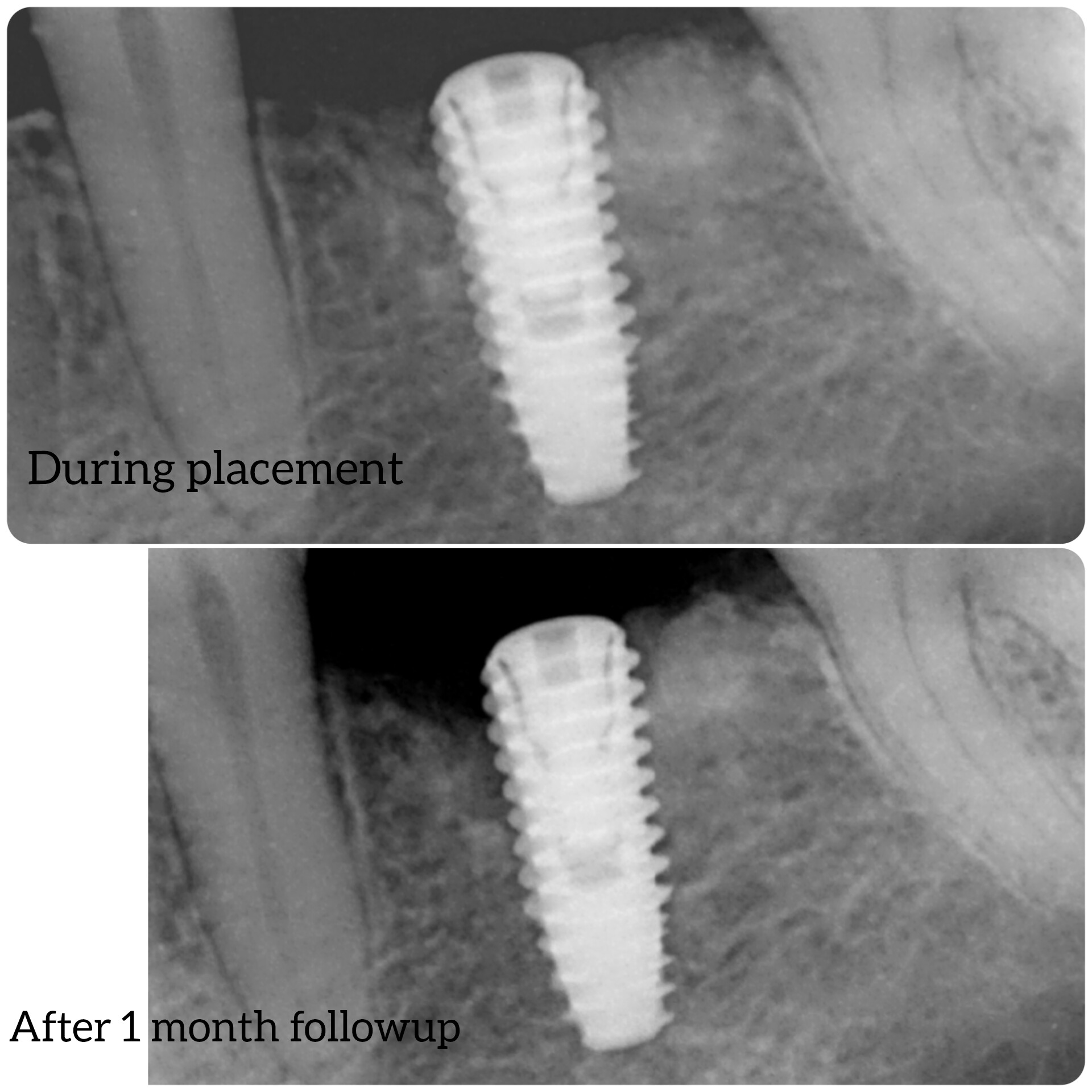
At this second interim stage, a statistically significant difference in bioactivity was not observed when comparing the Inion OptimaPLUS membrane with the GORE-TEX membrane, as measured by the primary endpoint (defect height) or secondary endpoints (histopathology, defect depth, width and horizontal thickness) of the trial.
On the basis of the results seen in the 31 patients completed to date, a statistically significant difference between the GORE-TEX and Inion membrane is unlikely to be observed on completion of the trial. However, completion of the trial is likely to provide support for the claim that there is no difference in bone growth between the Inion OptimaPLUS membrane and the GORE-TEX membrane in relation to the primary and secondary endpoints.
These interim results are from the first 31 patients (of a total of 40 recruited) to have completed the six-month clinical follow-up period. All of the remaining patients will have undergone the procedure by May 2008 and the study will be completed by the end of 2008, with the final report due in early 2009.
Chris Lee, CEO of Inion, said:
“So far in this trial, Inion OptimaPLUS has performed as well as the GORE-TEX® membrane in supporting new bone growth around dental implants, however, we have not seen evidence of bioactivity in this trial. Despite this, we still believe that by completing the trial we will be able to add to the product a claim that there is no difference in bone growth when compared to the GORE-TEX® membrane with the added benefit that the Inion OptimaPLUS™ membrane does not need to be removed with a further invasive procedure. Preclinical studies have shown that NMP[1] has the ability to accelerate bone growth, which, if it can be replicated in an appropriate human model, will add further value to the programme.
Source: Inion