Ostapek Vertebral Body Replacement Device Receives FDA Approval
Coligne AG, a leading innovator in the design and manufacture of spinal implants, has received U.S. Food and Drug Administration market clearance for its unique Ostapek composite VBR devices for treatment of the thoracic and lumbar spine.
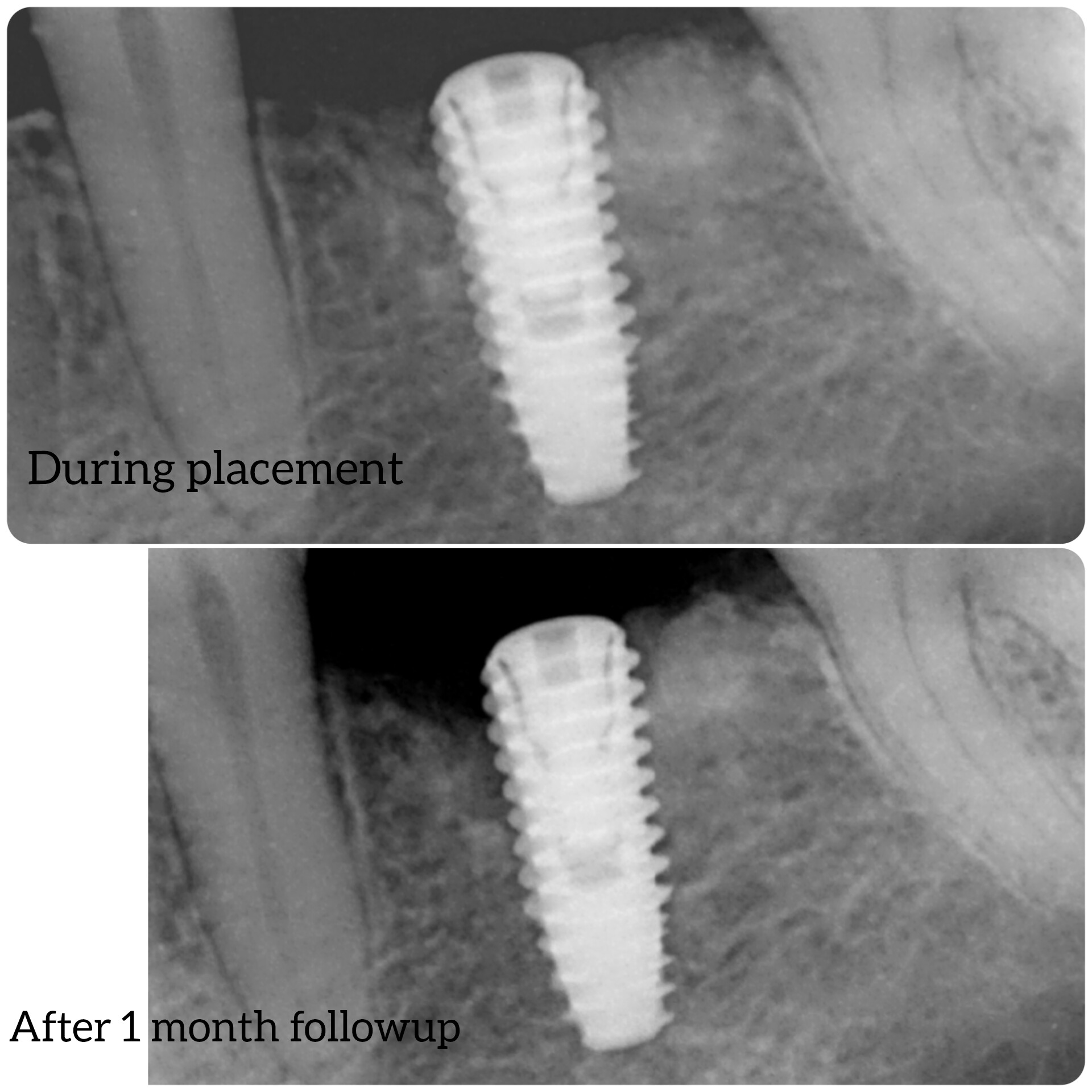
The first VBR (Vertebral Body Replacment) surgeries using Ostapek composite were performed by Dr. Alessandro Luzzati of Cremona, Italy, to treat cancer patients. Here the diseased vertebra is removed en bloc and then replaced by an autologous bone graft filled Ostapek cage construct, secured with spinal pedicle fixation.
“Our experience with many metal VBR systems made us understand that the best long term stabilization we can provide patients would be to help them build a suitable bridge of growing bone to replace the vertebra that has been removed and achieve a definitive anterior arthrodesis. The Ostapek composite VBR design increases the volume of bone graft possible in a modular and stable construct, and we are convinced this improves the patient’s possibilities to create a viable, stable and definitive fusionâ€, says Dr. Alessandro Luzzati.
Source:
coLigne AG
Tel.: 0041 43 343 8000
Fax: 0041 43 343 8009