Swedish Medical Body Requests More Information from Nobel Biocare
The NobelDirect and NobelPerfect saga continues.
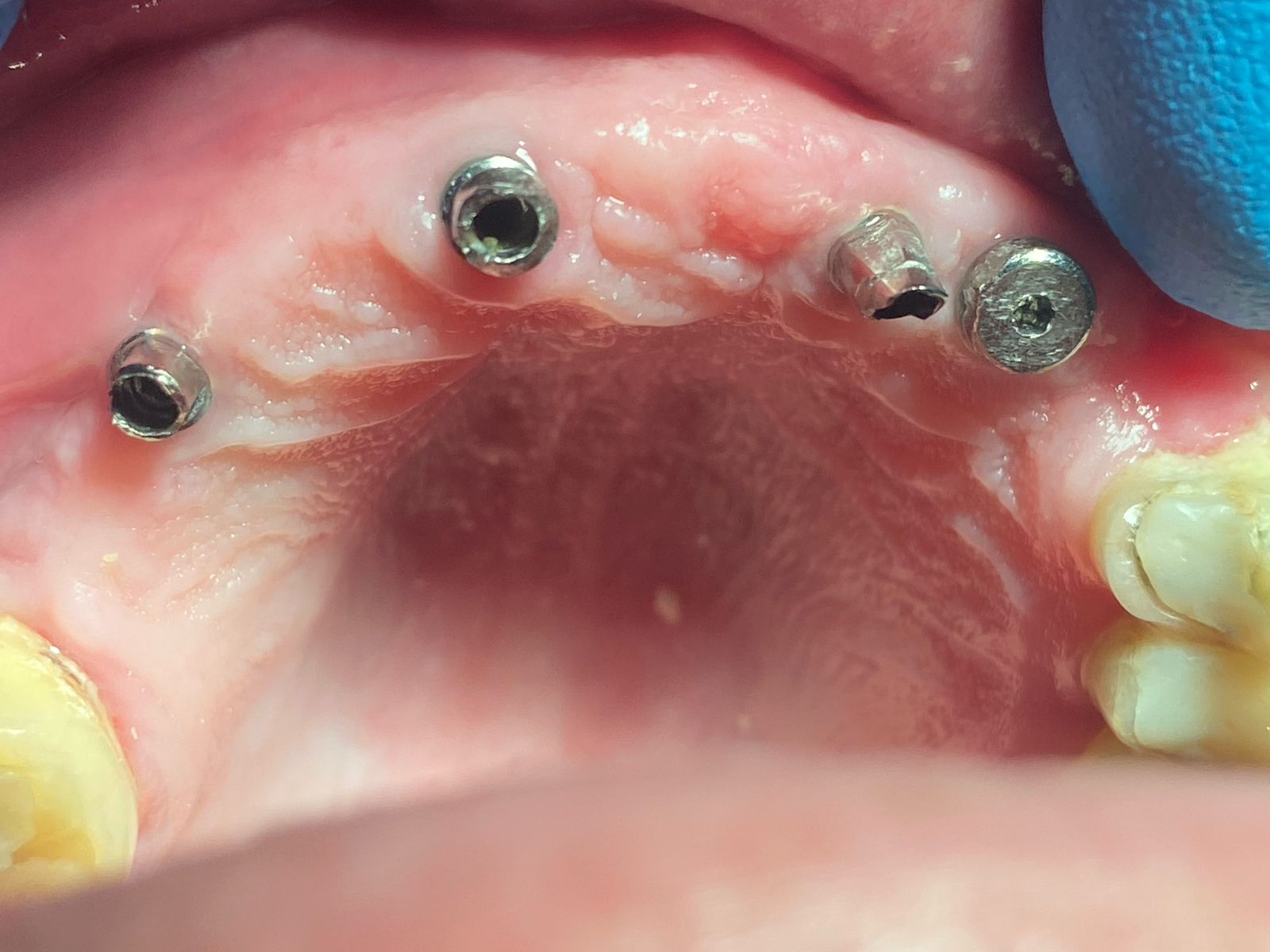
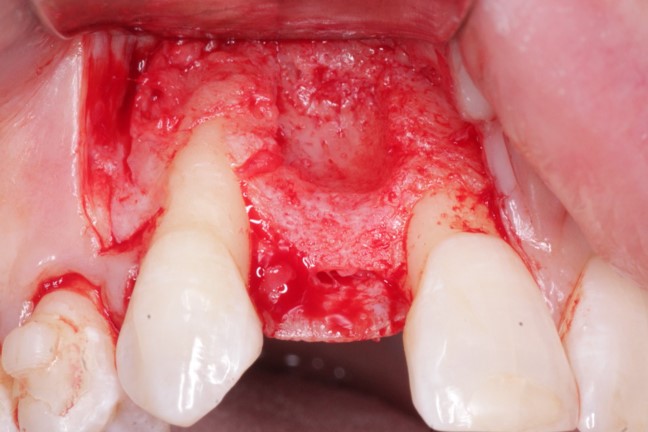
The Medical Products Agency has asked for additional information from Nobel Biocare AG on its dental implant products NobelDirect and NobelPerfect, reiterating that the group may not actively market the products before the Swedish medical watchdog approves.
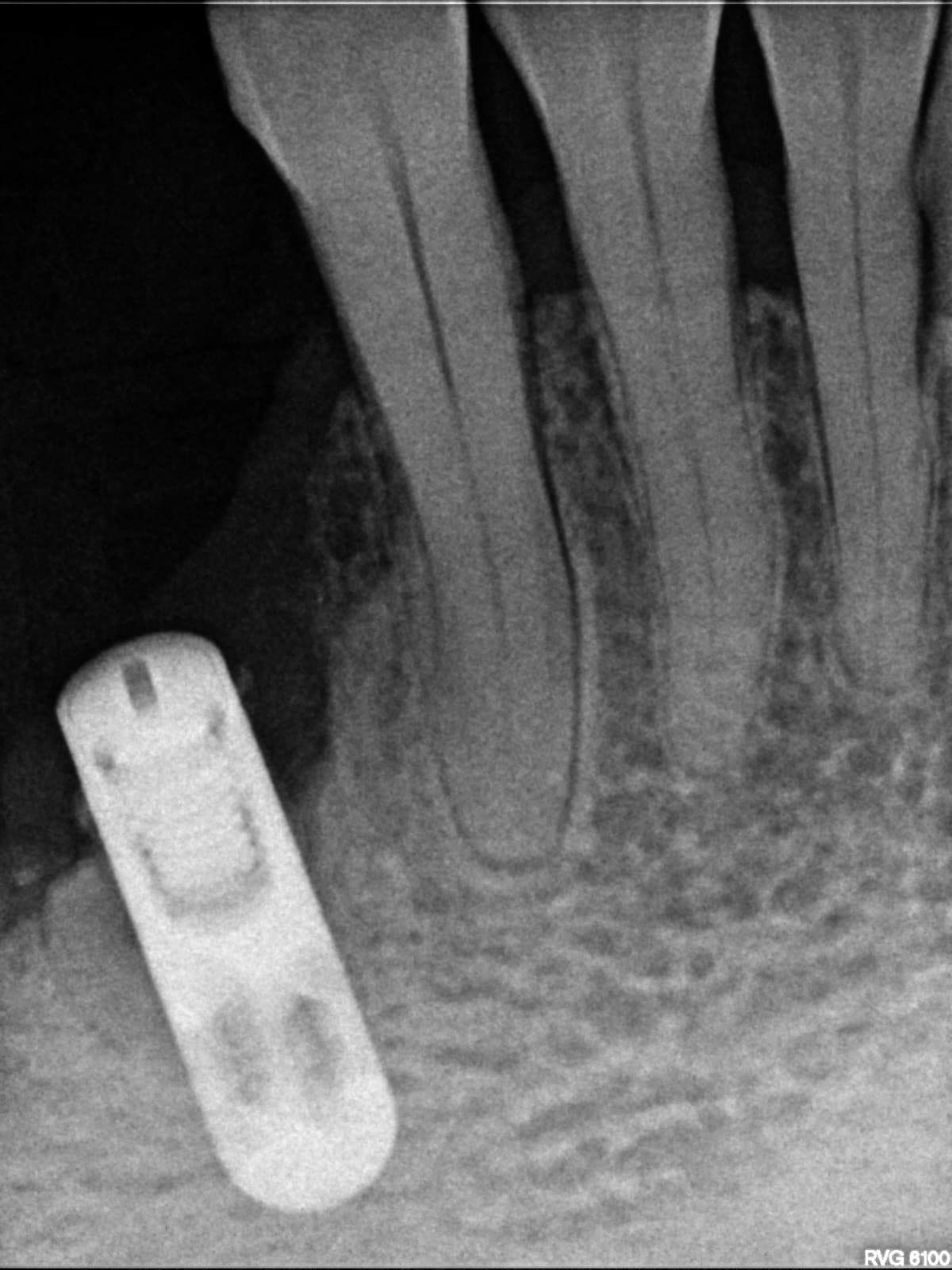
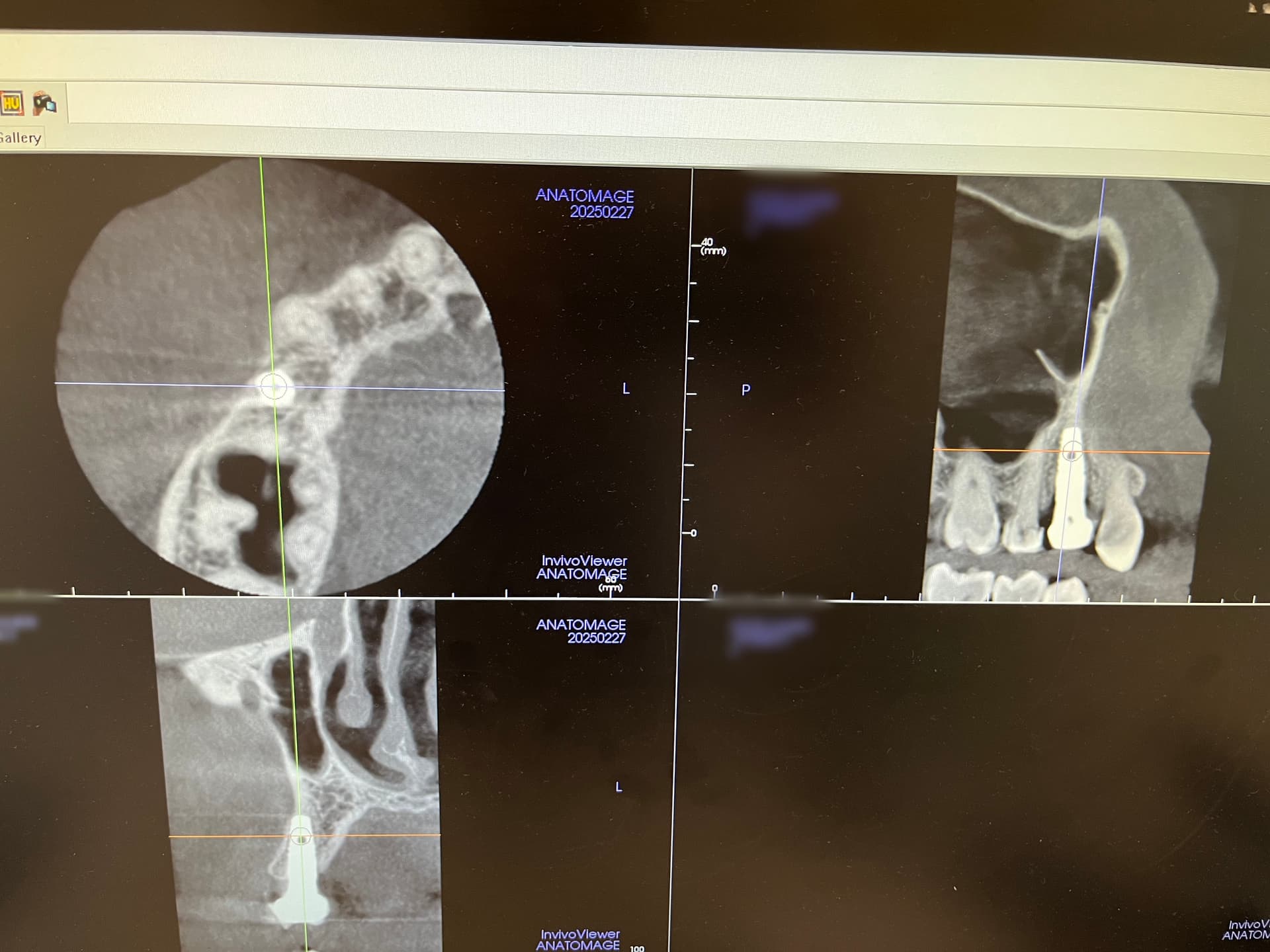
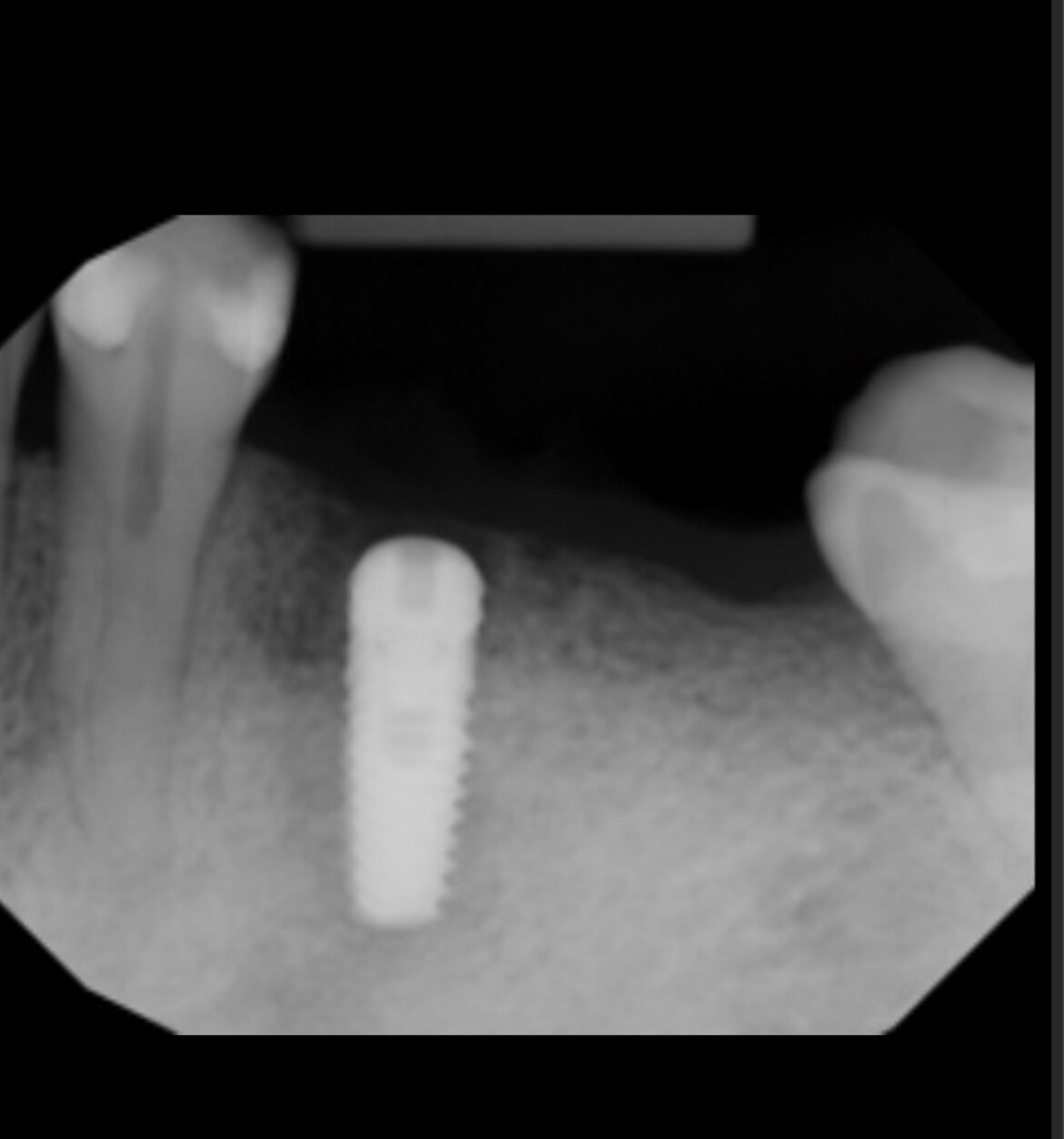
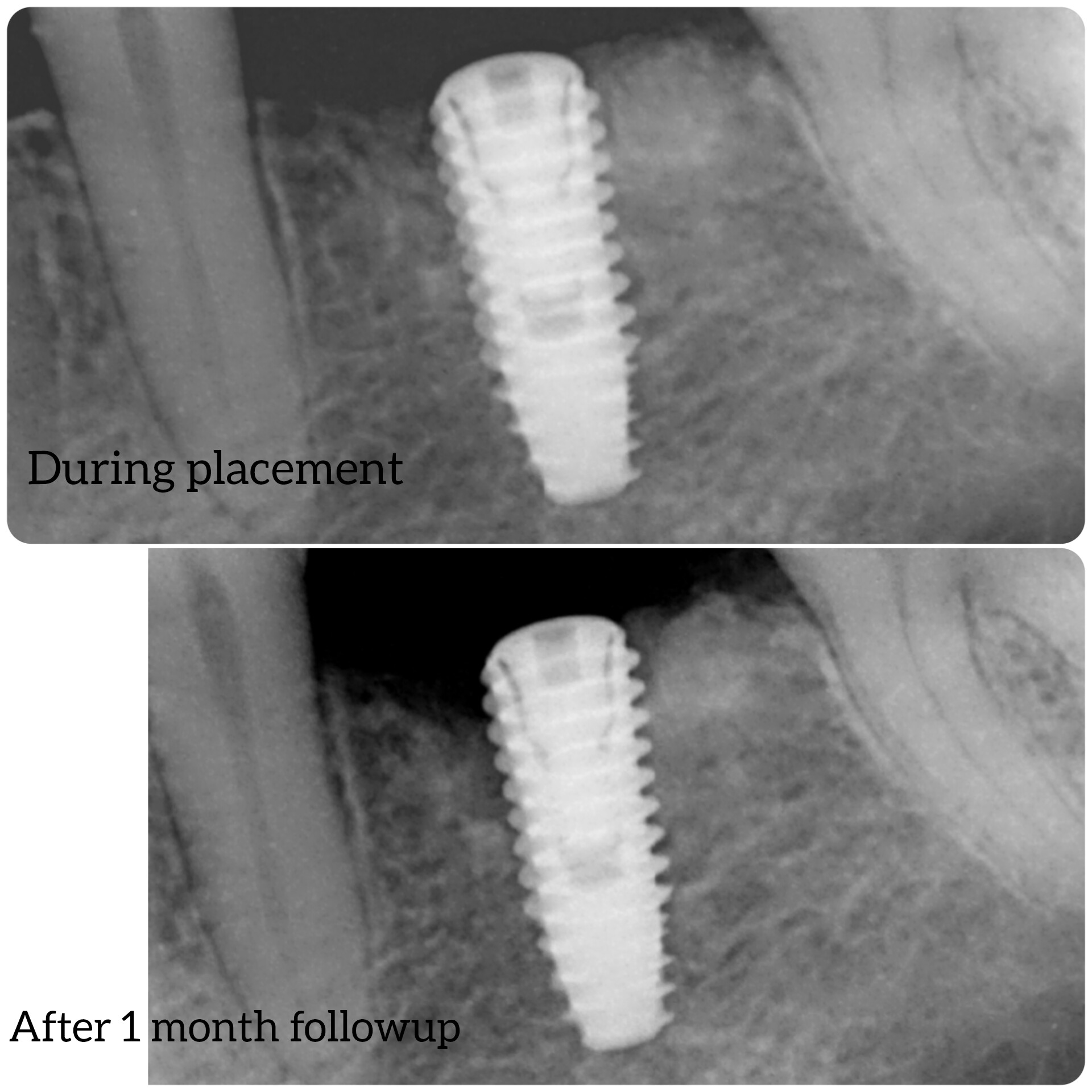
Earlier, the Medical Products Agency’s investigation of the dental implants indicated that the instructions for use for NobelDirect and NobelPerfect implants were inadequate.
The authority demanded that Nobel Biocare outline how they plan to disseminate information on improved instructions for the use of the dental implants.
A review of Nobel Biocare’s plan has resulted in a number of questions that Nobel Biocare has to respond to by March 13, 2007 at the latest.
Any thoughts on this latest news?
Source: Forbes.com